Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol
- PMID: 25915461
- PMCID: PMC6272178
- DOI: 10.3390/molecules20057454
Flavonoids from Sideritis Species: Human Monoamine Oxidase (hMAO) Inhibitory Activities, Molecular Docking Studies and Crystal Structure of Xanthomicrol
Abstract
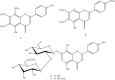
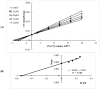
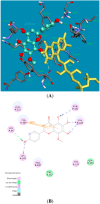
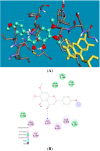
The inhibitory effects of flavonoids on monoamine oxidases (MAOs) have attracted great interest since alterations in monoaminergic transmission are reported to be related to neurodegenerative diseases such as Parkinson's and Alzheimer's diseases and psychiatric disorders such as depression and anxiety, thus MAOs may be considered as targets for the treatment of these multi-factorial diseases. In the present study, four Sideritis flavonoids, xanthomicrol (1), isoscutellarein 7-O-[6'''-O-acetyl-β-D-allopyranosyl-(1→2)]-β-D-glucopyranoside (2), isoscutellarein 7-O-[6'''-O-acetyl-β-D-allopyranosyl-(1→2)]-6''-O-acetyl-β-D-glucopyranoside (3) and salvigenin (4) were docked computationally into the active site of the human monoamine oxidase isoforms (hMAO-A and hMAO-B) and were also investigated for their hMAO inhibitory potencies using recombinant hMAO isoenzymes. The flavonoids inhibited hMAO-A selectively and reversibly in a competitive mode. Salvigenin (4) was found to be the most potent hMAO-A inhibitor, while xanthomicrol (1) appeared as the most selective hMAO-A inhibitor. The computationally obtained results were in good agreement with the corresponding experimental values. In addition, the x-ray structure of xanthomicrol (1) has been shown. The current work warrants further preclinical studies to assess the potential of xanthomicrol (1) and salvigenin (4) as new selective and reversible hMAO-A inhibitors for the treatment of depression and anxiety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



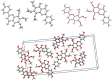
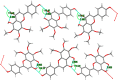
References
-
- Obon de Castro C., Rivera-Nunez D. Phanerogamarum Monographiae Tomus XXI: A Taxonomic Revision of the Section Sideritis (genus Sideritis) (Labiatae) J. Cramer; Berlin, Germany: 1994.
-
- Ezer N., Akcoş Y. Flavonoids from Sideritis lycia. J. Hacettepe Univ. Fac. Pharm. 1995;15:81–87.
-
- Gonzalez-Burgos E., Carretero M.E., Gomez-Serranillos M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities-a review. J. Ethnopharmacol. 1996;135:209–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

