Development and characterisation of a 3D multi-cellular in vitro model of normal human breast: a tool for cancer initiation studies
- PMID: 25915532
- PMCID: PMC4537045
- DOI: 10.18632/oncotarget.3803
Development and characterisation of a 3D multi-cellular in vitro model of normal human breast: a tool for cancer initiation studies
Abstract
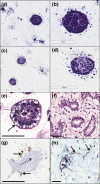
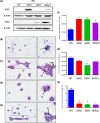
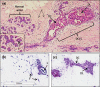
Multicellular 3-dimensional (3D) in vitro models of normal human breast tissue to study cancer initiation are required. We present a model incorporating three of the major functional cell types of breast, detail the phenotype and document our breast cancer initiation studies. Myoepithelial cells and fibroblasts were isolated and immortalised from breast reduction mammoplasty samples. Tri-cultures containing non-tumorigenic luminal epithelial cells HB2, or HB2 overexpressing different HER proteins, together with myoepithelial cells and fibroblasts were established in collagen I. Phenotype was assessed morphologically and immunohistochemically and compared to normal breast tissue. When all three cell types were present, polarised epithelial structures with lumens and basement membrane production were observed, akin to normal human breast tissue. Overexpression of HER2 or HER2/3 caused a significant increase in size, while HER2 overexpression resulted in development of a DCIS-like phenotype. In summary, we have developed a 3D tri-cellular model of normal human breast, amenable to comparative analysis after genetic manipulation and with potential to dissect the mechanisms behind the early stages of breast cancer initiation.
Keywords: 3D cell culture; HER2; breast.
Conflict of interest statement
The authors declare they have no conflicts of interest
Figures

Similar articles
-
A 3D in vitro model of the human breast duct: a method to unravel myoepithelial-luminal interactions in the progression of breast cancer.Breast Cancer Res. 2017 Apr 21;19(1):50. doi: 10.1186/s13058-017-0843-4. Breast Cancer Res. 2017. PMID: 28427436 Free PMC article.
-
Novel multicellular organotypic models of normal and malignant breast: tools for dissecting the role of the microenvironment in breast cancer progression.Breast Cancer Res. 2009;11(1):R3. doi: 10.1186/bcr2218. Epub 2009 Jan 19. Breast Cancer Res. 2009. PMID: 19152687 Free PMC article.
-
Modeling breast acini in tissue culture for detection of malignant phenotype reversion to non-malignant phenotype.Iran Biomed J. 2009 Oct;13(4):191-8. Iran Biomed J. 2009. PMID: 19946344
-
Human models of breast cancer.Cancer Surv. 1993;16:59-78. Cancer Surv. 1993. PMID: 8348539 Review.
-
Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures.Annu Rev Pathol. 2008;3:313-39. doi: 10.1146/annurev.pathmechdis.3.121806.151526. Annu Rev Pathol. 2008. PMID: 18039125 Review.
Cited by
-
Microdissected "cuboids" for microfluidic drug testing of intact tissues.Lab Chip. 2021 Jan 5;21(1):122-142. doi: 10.1039/d0lc00801j. Lab Chip. 2021. PMID: 33174580 Free PMC article.
-
Establishment of a normal-derived estrogen receptor-positive cell line comparable to the prevailing human breast cancer subtype.Oncotarget. 2017 Feb 7;8(6):10580-10593. doi: 10.18632/oncotarget.14554. Oncotarget. 2017. PMID: 28076334 Free PMC article.
-
Integrins as architects of cell behavior.Mol Biol Cell. 2016 Oct 1;27(19):2885-8. doi: 10.1091/mbc.E15-06-0369. Mol Biol Cell. 2016. PMID: 27687254 Free PMC article. Review.
-
In vitro models for head and neck cancer: Current status and future perspective.Front Oncol. 2022 Aug 3;12:960340. doi: 10.3389/fonc.2022.960340. eCollection 2022. Front Oncol. 2022. PMID: 35992863 Free PMC article. Review.
-
A microfluidic platform for functional testing of cancer drugs on intact tumor slices.Lab Chip. 2020 May 7;20(9):1658-1675. doi: 10.1039/c9lc00811j. Epub 2020 Apr 9. Lab Chip. 2020. PMID: 32270149 Free PMC article.
References
-
- Dhimolea E, Maffini MV, Soto AM, Sonnenschein C. The role of collagen reorganization on mammary epithelial morphogenesis in a 3D culture model. Biomaterials. 2010;31:3622–3630. - PubMed
-
- Krause S, Maffini MV, Soto AM, Sonnenschein C. A Novel 3D In Vitro Culture Model to Study Stromal–Epithelial Interactions in the Mammary Gland. Tissue Engineering Part C: Methods. 2008;14:261–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

