Hypoglycosylated hFSH Has Greater Bioactivity Than Fully Glycosylated Recombinant hFSH in Human Granulosa Cells
- PMID: 25915568
- PMCID: PMC4454802
- DOI: 10.1210/jc.2015-1317
Hypoglycosylated hFSH Has Greater Bioactivity Than Fully Glycosylated Recombinant hFSH in Human Granulosa Cells
Abstract
Context: Previous studies suggest that aging in women is associated with a reduction in hypoglycosylated forms of FSH.
Objective: Experiments were performed to determine whether glycosylation of the FSHβ subunit modulates the biological activity of FSH in human granulosa cells.
Design and setting: Recombinant human FSH (hFSH) derived from GH3 pituitary cells was purified into fractions containing hypoglycosylated hFSH(21/18) and fully glycosylated hFSH(24). The response to FSH glycoforms was evaluated using the well-characterized, FSH-responsive human granulosa cell line, KGN at an academic medical center.
Interventions: Granulosa cells were treated with increasing concentrations of fully- or hypoglycosylated FSH glycoforms for periods up to 48 hours.
Main outcome measure(s): The main outcomes were indices of cAMP-dependent cell signaling and estrogen and progesterone synthesis.
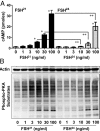
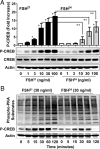
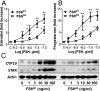
Results: We observed that hypoglycosylated FSH(21/18) was significantly more effective than fully glycosylated FSH(24) at stimulating cAMP accumulation, protein kinase A (PKA) activity, and cAMP response element binding protein (CREB) (S133) phosphorylation. FSH(21/18) was also much more effective than hFSH(24) on the stimulation CREB-response element-mediated transcription, expression of aromatase and STAR proteins, and synthesis of estrogen and progesterone. Adenoviral-mediated expression of the endogenous inhibitor of PKA, inhibited FSH(21/18)- and FSH(24)-stimulated CREB phosphorylation, and steroidogenesis.
Conclusions: Hypoglycosylated FSH(21/18) has greater bioactivity than fully glycosylated hFSH(24), suggesting that age-dependent decreases in hypoglycosylated hFSH contribute to reduced ovarian responsiveness. Hypoglycosylated FSH may be useful in follicle stimulation protocols for older patients using assisted reproduction technologies.
Figures


References
-
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994;8(6):722–731. - PubMed
-
- Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 1994;269(19):14015–14020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

