Neurovascular crosstalk between interneurons and capillaries is required for vision
- PMID: 25915585
- PMCID: PMC4497761
- DOI: 10.1172/JCI80297
Neurovascular crosstalk between interneurons and capillaries is required for vision
Abstract
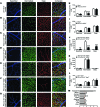
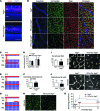
Functional interactions between neurons, vasculature, and glia within neurovascular units are critical for maintenance of the retina and other CNS tissues. For example, the architecture of the neurosensory retina is a highly organized structure with alternating layers of neurons and blood vessels that match the metabolic demand of neuronal activity with an appropriate supply of oxygen within perfused blood. Here, using murine genetic models and cell ablation strategies, we have demonstrated that a subset of retinal interneurons, the amacrine and horizontal cells, form neurovascular units with capillaries in 2 of the 3 retinal vascular plexuses. Moreover, we determined that these cells are required for generating and maintaining the intraretinal vasculature through precise regulation of hypoxia-inducible and proangiogenic factors, and that amacrine and horizontal cell dysfunction induces alterations to the intraretinal vasculature and substantial visual deficits. These findings demonstrate that specific retinal interneurons and the intraretinal vasculature are highly interdependent, and loss of either or both elicits profound effects on photoreceptor survival and function.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

