Tissue stiffness dictates development, homeostasis, and disease progression
- PMID: 25915734
- PMCID: PMC4594591
- DOI: 10.1080/15476278.2015.1019687
Tissue stiffness dictates development, homeostasis, and disease progression
Abstract
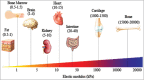
Tissue development is orchestrated by the coordinated activities of both chemical and physical regulators. While much attention has been given to the role that chemical regulators play in driving development, researchers have recently begun to elucidate the important role that the mechanical properties of the extracellular environment play. For instance, the stiffness of the extracellular environment has a role in orienting cell division, maintaining tissue boundaries, directing cell migration, and driving differentiation. In addition, extracellular matrix stiffness is important for maintaining normal tissue homeostasis, and when matrix mechanics become imbalanced, disease progression may ensue. In this article, we will review the important role that matrix stiffness plays in dictating cell behavior during development, tissue homeostasis, and disease progression.
Keywords: ECM, Extracellular matrix; EPC, Endothelial progenitor cell; FA, Focal adhesion; FAK, Focal adhesion kinase; LOX, Lysyl oxidase; MKL1, Megakaryoblastic leukemia factor-1; MMP, Matrix metalloproteinase; MSC, Mesenchymal stem cell; ROCK, Rho-associated protein kinase; VSMC, Vascular smooth muscle cell.; cancer; extracellular matrix; fibrosis; stiffness; tissue development; tissue homeostasis.
Figures

References
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 1996; 12:463-518; PMID:8970735; http://dx.doi.org/10.1146/annurev.cellbio.12.1.463 - DOI - PubMed
-
- Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol 2006; 18:541-8; PMID:16905303; http://dx.doi.org/10.1016/j.ceb.2006.08.004 - DOI - PubMed
-
- Kumar A, Gupta T, Berzsenyi S, Giangrande A. N-cadherin negatively regulates collective drosophila glial migration via actin cytoskeleton remodeling. J Cell Sci 2015; 128:900-12; PMID:25593128 - PubMed
-
- Podkowa M, Christova T, Zhao X, Jian Y, Attisano L. p21-Activated kinase (PAK) is required for bone morphogenetic protein (BMP)-induced dendritogenesis in cortical neurons. Mol Cellular Neurosci 2013; 57:83-92; PMID:24141051; http://dx.doi.org/10.1016/j.mcn.2013.10.005 - DOI - PubMed
-
- Morris HT, Machesky LM. Actin cytoskeletal control during epithelial to mesenchymal transition: focus on the pancreas and intestinal tract. British J Cancer 2015; 112:613-20; PMID:25611303; http://dx.doi.org/10.1038/bjc.2014.658 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
