Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers
- PMID: 25915810
- PMCID: PMC4532305
- DOI: 10.1038/bmt.2015.74
Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers
Abstract
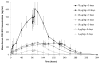
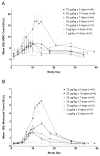
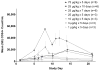
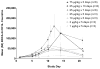
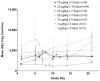
Fms-like tyrosine kinase-3 ligand (Flt3L) uniquely binds the Flt3 (CD135) receptor expressed on hematopoietic stem cells (HSCs), early progenitor cells, immature thymocytes and steady-state dendritic cells (DCs) and induces their proliferation, differentiation, development and mobilization in the bone marrow, peripheral blood and lymphoid organs. CDX-301 has an identical amino-acid sequence and comparable biological activity to the previously tested rhuFlt3L, which ceased clinical development over a decade ago. This Phase 1 trial assessed the safety, pharmacokinetic, pharmacodynamic and immunologic profile of CDX-301, explored alternate dosing regimens and examined the impact of rhuFlt3L on key immune cell subsets. Thirty healthy volunteers received CDX-301 (1-75 μg/kg/day) over 5-10 days. One event of Grade 3 community-acquired pneumonia occurred. There were no other infections, dose-limiting toxicities or serious adverse events. CDX-301 resulted in effective peripheral expansion of monocytes, hematopoietic stem and progenitor cells and key subsets of myeloid DCs and plasmacytoid DCs, with no clear effect on regulatory T cells. These data from healthy volunteers support the potential for CDX-301, as monotherapy or in combination with other agents, in various indications including allogeneic HSC transplantation and immunotherapy, but the effects of CDX-301 will need to be investigated in each of these patient populations.
Conflict of interest statement
J.G., T.H., H.C.M., M.Y., T.D., and T.K. are employed by and hold stock options in Celldex Therapeutics, Inc. S.S. is on the board of directors and holds stock in Ariad. The remaining authors have no conflict of interest to disclose.
Figures

References
-
- Bertho JM, Chapel A, Loilleux S, Frick J, Aigueperse J, Gorin NC, et al. CD135 (Flk2/Flt3) expression by human thymocytes delineates a possible role of FLT3-ligand in T-cell precursor proliferation and differentiation. Scandinavian journal of immunology. 2000;52:53–61. - PubMed
-
- Gabbianelli M, Pelosi E, Montesoro E, Valtieri M, Luchetti L, Samoggia P, et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–1670. - PubMed
-
- Gasparetto C, Gasparetto M, Morse M, Rooney B, Vredenburgh JJ, Long GD, et al. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine. 2002;18:8–19. - PubMed
-
- Chao N, Litzow MR, Geller RB, Korbling M, Doroshow JH, Fay J, et al. Randomized Phase II Study of FLT3 Ligand (MOBIST™) in Combination With GM-CSF or G-CSF for Mobiilization of Peripheral Blood Progenitor Cells in Patients with Breast Cancer. American Society of Hematology Meeting Abstract. 1999;2954
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

