CD2v Interacts with Adaptor Protein AP-1 during African Swine Fever Infection
- PMID: 25915900
- PMCID: PMC4411086
- DOI: 10.1371/journal.pone.0123714
CD2v Interacts with Adaptor Protein AP-1 during African Swine Fever Infection
Abstract
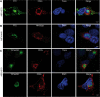
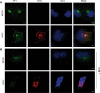
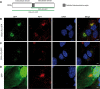
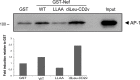
African swine fever virus (ASFV) CD2v protein is believed to be involved in virulence enhancement, viral hemadsorption, and pathogenesis, although the molecular mechanisms of the function of this viral protein are still not fully understood. Here we describe that CD2v localized around viral factories during ASFV infection, suggesting a role in the generation and/or dynamics of these viral structures and hence in disturbing cellular traffic. We show that CD2v targeted the regulatory trans-Golgi network (TGN) protein complex AP-1, a key element in cellular traffic. This interaction was disrupted by brefeldin A even though the location of CD2v around the viral factory remained unchanged. CD2v-AP-1 binding was independent of CD2v glycosylation and occurred on the carboxy-terminal part of CD2v, where a canonical di-Leu motif previously reported to mediate AP-1 binding in eukaryotic cells, was identified. This motif was shown to be functionally interchangeable with the di-Leu motif present in HIV-Nef protein in an AP-1 binding assay. However, we demonstrated that it was not involved either in CD2v cellular distribution or in CD2v-AP-1 binding. Taken together, these findings shed light on CD2v function during ASFV infection by identifying AP-1 as a cellular factor targeted by CD2v and hence elucidate the cellular pathways used by the virus to enhance infectivity.
Conflict of interest statement
Figures



Similar articles
-
The CD2v protein of African swine fever virus interacts with the actin-binding adaptor protein SH3P7.J Gen Virol. 2004 Jan;85(Pt 1):119-130. doi: 10.1099/vir.0.19435-0. J Gen Virol. 2004. PMID: 14718626
-
African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells.Viruses. 2021 Jul 28;13(8):1480. doi: 10.3390/v13081480. Viruses. 2021. PMID: 34452346 Free PMC article.
-
From structure prediction to function: defining the domain on the African swine fever virus CD2v protein required for binding to erythrocytes.mBio. 2025 Feb 5;16(2):e0165524. doi: 10.1128/mbio.01655-24. Epub 2024 Dec 17. mBio. 2025. PMID: 39688401 Free PMC article.
-
Processing and localization of the african swine fever virus CD2v transmembrane protein.J Virol. 2011 Apr;85(7):3294-305. doi: 10.1128/JVI.01994-10. Epub 2011 Jan 19. J Virol. 2011. PMID: 21248037 Free PMC article.
-
African swine fever virus controls the host transcription and cellular machinery of protein synthesis.Virus Res. 2013 Apr;173(1):58-75. doi: 10.1016/j.virusres.2012.10.025. Epub 2012 Nov 12. Virus Res. 2013. PMID: 23154157 Review.
Cited by
-
Deletion of the H108R Gene Reduces Virulence of the Pandemic Eurasia Strain of African Swine Fever Virus with Surviving Animals Being Protected against Virulent Challenge.J Virol. 2022 Jul 27;96(14):e0054522. doi: 10.1128/jvi.00545-22. Epub 2022 Jul 6. J Virol. 2022. PMID: 35862691 Free PMC article.
-
Computational insights in CD58 binding to ASFV CD2v and in silico optimization of nanobody designs against the interface.Open Vet J. 2025 Jan;15(1):348-387. doi: 10.5455/OVJ.2025.v15.i1.32. Epub 2025 Jan 31. Open Vet J. 2025. PMID: 40092189 Free PMC article.
-
Development and characterization of monoclonal antibodies against the extracellular domain of African swine fever virus structural protein, CD2v.Front Microbiol. 2022 Nov 18;13:1056117. doi: 10.3389/fmicb.2022.1056117. eCollection 2022. Front Microbiol. 2022. PMID: 36466651 Free PMC article.
-
Spatiotemporally Orchestrated Interactions between Viral and Cellular Proteins Involved in the Entry of African Swine Fever Virus.Viruses. 2021 Dec 13;13(12):2495. doi: 10.3390/v13122495. Viruses. 2021. PMID: 34960765 Free PMC article. Review.
-
The AP-1 adaptor complex is essential for intracellular trafficking of the ORF2 capsid protein and assembly of Hepatitis E virus.Cell Mol Life Sci. 2024 Aug 9;81(1):335. doi: 10.1007/s00018-024-05367-0. Cell Mol Life Sci. 2024. PMID: 39117755 Free PMC article.
References
-
- Dixon L, Alonso C, Escribano JM, Martins C, Revilla Y, Salas ML, et al. (2012) The Asfarviridae. In virus Taxonomy. Ninth Report of the International Comittee on Taxonomy of Viruses. Elsevier: 153–162.
-
- Dixon LK, Abrams CC, Bowick G, Goatley LC, Kay-Jackson PC, Champan D, et al. (2004) African swine fever virus proteins involved in evading host defence systems. Veterinary immunology and immunopathology 100: 117–134. - PubMed
-
- Granja AG, Perkins ND, Revilla Y (2008) A238L inhibits NF-ATc2, NF-kappa B, and c-Jun activation through a novel mechanism involving protein kinase C-theta-mediated up-regulation of the amino-terminal transactivation domain of p300. J Immunol 180: 2429–2442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

