Clinical efficacy and safety of Ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials
- PMID: 25915909
- PMCID: PMC4411142
- DOI: 10.1371/journal.pone.0124587
Clinical efficacy and safety of Ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Randomized clinical trials (RCTs) about Ezetimibe's efficacy on patient-oriented outcomes have given discordant results. The aim of this study was to determine the net effect of Ezetimibe and of the widely marketed combination, Ezetimibe+simvastatin, on mortality and morbidity outcomes.
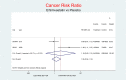
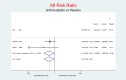
Methods and findings: We searched for RCT on Ezetimibe using MEDLINE, CCTR, EMBASE, ClinicalTrials.gov databases up to December 2013, Merck and Novartis online registers, and personal communications. Two authors independently selected trials fulfilling these criteria: RCTs comparing Ezetimibe±statin or another lipid-lowering drug against placebo, or against the same lipid-lowering drug at the same dosage, with a follow-up at least 24 weeks and one or more of these outcomes: all-cause mortality, cardiovascular (CV) mortality, stroke, myocardial infarction (MI), cancer, serious adverse events (SAEs); we assessed the risk of bias using the Cochrane checklist. We extracted the data for major clinical events as a dichotomous measure, with the patient the unit of analysis. Pooled analysis was done with random and fixed effect based models. Trials comparing Ezetimibe plus a lipid-lowering drug against the same lipidlowering drug representing the net effect of Ezetimibe, showed a nonsignificant tendency toward damage for cancer, MI, stroke and SAEs. Ezetimibe+simvastatin vs. simvastatin alone showed a stronger tendency towards a higher risk for all-cause death (2.52; 0.65-9.74), CV death (3.04; 0.48-19.21), non-CV death (3.03; 0.12-73.50), MI (1.91; 0.42-8.70), stroke (2.38; 0.46-12.35), cancer (RR 11.11; 0.62-198.29), and SAEs (1.45; 0.95-2.23). Limitations include small numbers of events and inadequate power of the pooling. Trials comparing Ezetimibe+simvastatin vs placebo showed non-significant effects: MI (0.81; 0.66-1.00 p = 0.051), all-cause death (1.02; 0.95-1.09), CV death (0.91; 0.80-1.04), non-CV death (108; 0.99-1.18), stroke (0.86; 0.72-1.04), cancer (1.18; 0.80-1.74), SAEs (1.01; 0.96-1.06).
Conclusions: Ezetimibe±simvastatin had inconsistent effects on important outcomes. No firm conclusions are possible, but findings indicative of damage suggest much more selective use of Ezetimibe±simvastatin.
Conflict of interest statement
Figures












References
-
- Todd C. Villines TD, Stanek EJ, Devine PJ, Turco M, Miller M, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis). Final Results and the Impact of Medication Adherence, Dose, and Treatment Duration. J Am Coll Cardiol. 2010;55:2721–2726. 10.1016/j.jacc.2010.03.017 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

