REGULATOR OF BULB BIOGENESIS1 (RBB1) Is Involved in Vacuole Bulb Formation in Arabidopsis
- PMID: 25915922
- PMCID: PMC4411111
- DOI: 10.1371/journal.pone.0125621
REGULATOR OF BULB BIOGENESIS1 (RBB1) Is Involved in Vacuole Bulb Formation in Arabidopsis
Abstract
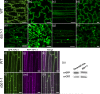
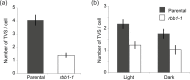
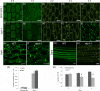
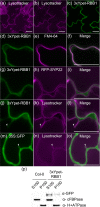
Vacuoles are dynamic compartments with constant fluctuations and transient structures such as trans-vacuolar strands and bulbs. Bulbs are highly dynamic spherical structures inside vacuoles that are formed by multiple layers of membranes and are continuous with the main tonoplast. We recently carried out a screen for mutants with abnormal trafficking to the vacuole or aberrant vacuole morphology. We characterized regulator of bulb biogenesis1-1 (rbb1-1), a mutant in Arabidopsis that contains increased numbers of bulbs when compared to the parental control. rbb1-1 mutants also contain fewer transvacuolar strands than the parental control, and we propose the hypothesis that the formation of transvacuolar strands and bulbs is functionally related. We propose that the bulbs may function transiently to accommodate membranes and proteins when transvacuolar strands fail to elongate. We show that RBB1 corresponds to a very large protein of unknown function that is specific to plants, is present in the cytosol, and may associate with cellular membranes. RBB1 is involved in the regulation of vacuole morphology and may be involved in the establishment or stability of trans-vacuolar strands and bulbs.
Conflict of interest statement
Figures


References
-
- Uemura T, Yoshimura SH, Takeyasu K, Sato MH. Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes to Cells. 2002;7(7):743–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

