Assessment of blood flow velocity and pulsatility in cerebral perforating arteries with 7-T quantitative flow MRI
- PMID: 25916399
- PMCID: PMC5008170
- DOI: 10.1002/nbm.3306
Assessment of blood flow velocity and pulsatility in cerebral perforating arteries with 7-T quantitative flow MRI
Abstract
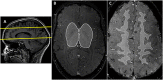
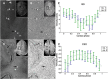
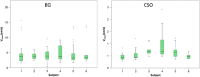
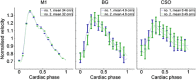
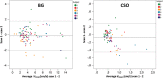
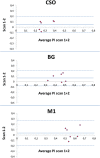
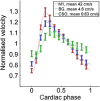
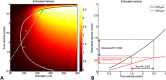
Thus far, blood flow velocity measurements with MRI have only been feasible in large cerebral blood vessels. High-field-strength MRI may now permit velocity measurements in much smaller arteries. The aim of this proof of principle study was to measure the blood flow velocity and pulsatility of cerebral perforating arteries with 7-T MRI. A two-dimensional (2D), single-slice quantitative flow (Qflow) sequence was used to measure blood flow velocities during the cardiac cycle in perforating arteries in the basal ganglia (BG) and semioval centre (CSO), from which a mean normalised pulsatility index (PI) per region was calculated (n = 6 human subjects, aged 23-29 years). The precision of the measurements was determined by repeated imaging and performance of a Bland-Altman analysis, and confounding effects of partial volume and noise on the measurements were simulated. The median number of arteries included was 14 in CSO and 19 in BG. In CSO, the average velocity per volunteer was in the range 0.5-1.0 cm/s and PI was 0.24-0.39. In BG, the average velocity was in the range 3.9-5.1 cm/s and PI was 0.51-0.62. Between repeated scans, the precision of the average, maximum and minimum velocity per vessel decreased with the size of the arteries, and was relatively low in CSO and BG compared with the M1 segment of the middle cerebral artery. The precision of PI per region was comparable with that of M1. The simulations proved that velocities can be measured in vessels with a diameter of more than 80 µm, but are underestimated as a result of partial volume effects, whilst pulsatility is overestimated. Blood flow velocity and pulsatility in cerebral perforating arteries have been measured directly in vivo for the first time, with moderate to good precision. This may be an interesting metric for the study of haemodynamic changes in aging and cerebral small vessel disease. © 2015 The Authors NMR in Biomedicine Published by John Wiley & Sons Ltd.
Keywords: MRI; Qflow; blood; brain; pulsatility; velocity.
© 2015 The Authors. NMR in Biomedicine Published by John Wiley & Sons Ltd.
Figures
References
-
- Mills S, Cain J, Purandare N, Jackson A. Biomarkers of cerebrovascular disease in dementia. Br. J. Radiol. 2007; 80 Spec No: S128–S145. - PubMed
-
- Henskens LHG, Kroon AA, van Oostenbrugge RJ, Gronenschild EHBM, Fuss‐Lejeune MMJJ, Hofman PAM, Lodder J, de Leeuw P. Increased aortic pulse wave velocity is associated with silent cerebral small‐vessel disease in hypertensive patients. Hypertension 2008; 52(6): 1120–1126. - PubMed
-
- Duprez DA, De Buyzere ML, Van den Noortgate N, Simoens J, Achten E, Clement DL, Afschrift M, Cohn JN. Relationship between periventricular or deep white matter lesions and arterial elasticity indices in very old people. Age Ageing, 2001; 30(4): 325–330. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

