Pharmacologic and clinical evaluation of posaconazole
- PMID: 25916666
- PMCID: PMC5125539
- DOI: 10.1586/17512433.2015.1034689
Pharmacologic and clinical evaluation of posaconazole
Abstract
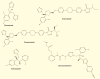
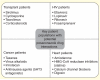
Posaconazole, a broad-spectrum triazole antifungal agent, is approved for the prevention of invasive aspergillosis and candidiasis in addition to the treatment of oropharyngeal candidiasis. There is evidence of efficacy in the treatment and prevention of rarer, more difficult-to-treat fungal infections. Posaconazole oral suspension solution has shown limitations with respect to fasting state absorption, elevated gastrointestinal pH and increased motility. The newly approved delayed-release oral tablet and intravenous solution formulations provide an attractive treatment option by reducing interpatient variability and providing flexibility in critically ill patients. On the basis of clinical experience and further clinical studies, posaconazole was found to be a valuable pharmaceutical agent for the treatment of life-threatening fungal infections. This review will examine the development history of posaconazole and highlight the most recent advances.
Keywords: antifungal; immunosuppression; invasive fungal infection; pharmacokinetics; pharmacology; posaconazole; triazole.
Figures


References
-
- Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. - PubMed
-
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93. - PubMed
-
- Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46(3):327–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
