Enterococcus faecalis Gelatinase Mediates Intestinal Permeability via Protease-Activated Receptor 2
- PMID: 25916983
- PMCID: PMC4468563
- DOI: 10.1128/IAI.00425-15
Enterococcus faecalis Gelatinase Mediates Intestinal Permeability via Protease-Activated Receptor 2
Abstract
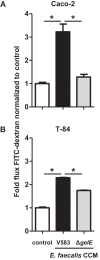
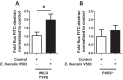
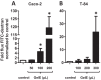
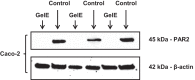
Microbial protease-mediated disruption of the intestinal epithelium is a potential mechanism whereby a dysbiotic enteric microbiota can lead to disease. This mechanism was investigated using the colitogenic, protease-secreting enteric microbe Enterococcus faecalis. Caco-2 and T-84 epithelial cell monolayers and the mouse colonic epithelium were exposed to concentrated conditioned media (CCM) from E. faecalis V583 and E. faecalis lacking the gelatinase gene (gelE). The flux of fluorescein isothiocyanate (FITC)-labeled dextran across monolayers or the mouse epithelium following exposure to CCM from parental or mutant E. faecalis strains indicated paracellular permeability. A protease-activated receptor 2 (PAR2) antagonist and PAR2-deficient (PAR2(-/-)) mice were used to investigate the role of this receptor in E. faecalis-induced permeability. Gelatinase (GelE) purified from E. faecalis V583 was used to confirm the ability of this protease to induce epithelial cell permeability and activate PAR2. The protease-mediated permeability of colonic epithelia from wild-type (WT) and PAR2(-/-) mice by fecal supernatants from ulcerative colitis patients was assessed. Secreted E. faecalis proteins induced permeability in epithelial cell monolayers, which was reduced in the absence of gelE or by blocking PAR2 activity. Secreted E. faecalis proteins induced permeability in the colonic epithelia of WT mice that was absent in tissues from PAR2(-/-) mice. Purified GelE confirmed the ability of this protease to induce epithelial cell permeability via PAR2 activation. Fecal supernatants from ulcerative colitis patients induced permeability in the colonic epithelia of WT mice that was reduced in tissues from PAR2(-/-) mice. Our investigations demonstrate that GelE from E. faecalis can regulate enteric epithelial permeability via PAR2.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

