Hydrogel-based Delivery of rhBMP-2 Improves Healing of Large Bone Defects Compared With Autograft
- PMID: 25917422
- PMCID: PMC4523508
- DOI: 10.1007/s11999-015-4312-z
Hydrogel-based Delivery of rhBMP-2 Improves Healing of Large Bone Defects Compared With Autograft
Abstract
Background: Autologous bone grafting remains the gold standard in the treatment of large bone defects but is limited by tissue availability and donor site morbidity. Recombinant human bone morphogenetic protein-2 (rhBMP-2), delivered with a collagen sponge, is clinically used to treat large bone defects and complications such as delayed healing or nonunion. For the same dose of rhBMP-2, we have shown that a hybrid nanofiber mesh-alginate (NMA-rhBMP-2) delivery system provides longer-term release and increases functional bone regeneration in critically sized rat femoral bone defects compared with a collagen sponge. However, no comparisons of healing efficiencies have been made thus far between this hybrid delivery system and the gold standard of using autograft.
Questions/purposes: We compared the efficacy of the NMA-rhBMP-2 hybrid delivery system to morselized autograft and hypothesized that the functional regeneration of large bone defects observed with sustained BMP delivery would be at least comparable to autograft treatment as measured by total bone volume and ex vivo mechanical properties.
Methods: Bilateral critically sized femoral bone defects in rats were treated with either live autograft or with the NMA-rhBMP-2 hybrid delivery system such that each animal received one treatment per leg. Healing was monitored by radiography and histology at 2, 4, 8, and 12 weeks. Defects were evaluated for bone formation by longitudinal micro-CT scans over 12 weeks (n = 14 per group). The bone volume, bone density, and the total new bone formed beyond 2 weeks within the defect were calculated from micro-CT reconstructions and values compared for the 2-, 4-, 8-, and 12-week scans within and across the two treatment groups. Two animals were used for bone labeling with subcutaneously injected dyes at 4, 8, and 12 weeks followed by histology at 12 weeks to identify incremental new bone formation. Functional recovery was measured by ex vivo biomechanical testing (n = 9 per group). Maximum torque and torsional stiffness calculated from torsion testing of the femurs at 12 weeks were compared between the two groups.
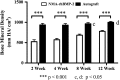
Results: The NMA-rhBMP-2 hybrid delivery system resulted in greater bone formation and improved biomechanical properties compared with autograft at 12 weeks. Comparing new bone volume within each group, the NMA-rhBMP-2-treated group had higher volume (p < 0.001) at 12 weeks (72.59 ± 18.34 mm(3)) compared with 8 weeks (54.90 ± 16.14) and 4 weeks (14.22 ± 9.59). The new bone volume was also higher at 8 weeks compared with 4 weeks (p < 0.001). The autograft group showed higher (p <0.05) new bone volume at 8 weeks (11.19 ± 8.59 mm(3)) and 12 weeks (14.64 ± 10.36) compared with 4 weeks (5.15 ± 4.90). Between groups, the NMA-rhBMP-2-treated group had higher (p < 0.001) new bone volume than the autograft group at both 8 and 12 weeks. Local mineralized matrix density in the NMA-rhBMP-2-treated group was lower than that of the autograft group at all time points (p < 0.001). Presence of nuclei within the lacunae of the autograft and early appositional bone formation seen in representative histology sections suggested that the bone grafts remained viable and were functionally engrafted within the defect. The bone label distribution from representative sections also revealed more diffuse mineralization in the defect in the NMA-rhBMP-2-treated group, whereas more localized distribution of new mineral was seen at the edges of the graft pieces in the autograft group. The NMA-rhBMP-2-treated group also revealed higher torsional stiffness (0.042 ± 0.019 versus 0.020 ± 0.022 N-m/°; p = 0.037) and higher maximum torque (0.270 ± 0.108 versus 0.125 ± 0.137 N-m; p = 0.024) compared with autograft.
Conclusions: The NMA-rhBMP-2 hybrid delivery system improved bone formation and restoration of biomechanical function of rat segmental bone defects compared with autograft treatment.
Clinical relevance: Delivery systems that allow prolonged availability of BMP may provide an effective clinical alternative to autograft treatment for repair of segmental bone defects. Future studies in a large animal model comparing mixed cortical-trabecular autograft and the NMA-rhBMP-2 hybrid delivery system are the next step toward clinical translation of this approach.
Figures







Similar articles
-
Vancomycin-bearing synthetic bone graft delivers rhBMP-2 and promotes healing of critical rat femoral segmental defects.Clin Orthop Relat Res. 2014 Dec;472(12):4015-23. doi: 10.1007/s11999-014-3841-1. Epub 2014 Aug 7. Clin Orthop Relat Res. 2014. PMID: 25099263 Free PMC article.
-
Treatment of critically sized femoral defects with recombinant BMP-2 delivered by a modified mPEG-PLGA biodegradable thermosensitive hydrogel.BMC Musculoskelet Disord. 2016 Jul 15;17:286. doi: 10.1186/s12891-016-1131-7. BMC Musculoskelet Disord. 2016. PMID: 27421654 Free PMC article.
-
Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2.Acta Biomater. 2017 Feb;49:101-112. doi: 10.1016/j.actbio.2016.12.012. Epub 2016 Dec 8. Acta Biomater. 2017. PMID: 27940197 Free PMC article.
-
Stem cell therapy: is there a future for reconstruction of large bone defects?Injury. 2016 Jan;47 Suppl 1:S47-51. doi: 10.1016/S0020-1383(16)30012-2. Injury. 2016. PMID: 26768292 Review.
-
Autologous Bone Graft in Foot and Ankle Surgery.Foot Ankle Clin. 2016 Dec;21(4):825-837. doi: 10.1016/j.fcl.2016.07.007. Foot Ankle Clin. 2016. PMID: 27871415 Review.
Cited by
-
Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones.J Biomed Opt. 2018 Sep;23(9):1-11. doi: 10.1117/1.JBO.23.9.097004. J Biomed Opt. 2018. PMID: 30264554 Free PMC article.
-
Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration.Stem Cells Transl Med. 2019 Jun;8(6):575-585. doi: 10.1002/sctm.18-0141. Epub 2019 Jan 21. Stem Cells Transl Med. 2019. PMID: 30666821 Free PMC article.
-
* Skeletal Myoblast-Seeded Vascularized Tissue Scaffolds in the Treatment of a Large Volumetric Muscle Defect in the Rat Biceps Femoris Muscle.Tissue Eng Part A. 2017 Sep;23(17-18):989-1000. doi: 10.1089/ten.TEA.2016.0523. Epub 2017 Aug 23. Tissue Eng Part A. 2017. PMID: 28372522 Free PMC article.
-
Functionalized Scaffold and Barrier Membrane with Anti-BMP-2 Monoclonal Antibodies for Alveolar Ridge Preservation in a Canine Model.Biomed Res Int. 2020 Sep 22;2020:6153724. doi: 10.1155/2020/6153724. eCollection 2020. Biomed Res Int. 2020. PMID: 33029518 Free PMC article.
-
Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration.Pharm Res. 2017 Jun;34(6):1152-1170. doi: 10.1007/s11095-017-2147-x. Epub 2017 Mar 24. Pharm Res. 2017. PMID: 28342056 Free PMC article. Review.
References
-
- Albert A, Leemrijse T, Druez V, Delloye C, Cornu O. Are bone autografts still necessary in 2006? A three-year retrospective study of bone grafting. Acta Orthop Belg. 2006;72:734–740. - PubMed
-
- Anderson KJ. The behavior of autogenous and homogenous bone transplants in the anterior chamber of the rat’s eye. A histological study of the effect of the size of the implant. J Bone Joint Surg Am. 1961;43:980–995. - PubMed
-
- Anderson KJ, Schmidt J, Lecocq JF. The effect of particle size of the heterogenous-bone transplant on the host-tissue vascular penetration. J Bone Joint Surg Am. 1959;41:1455–1468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

