Galanin modulates the neural niche to favour perineural invasion in head and neck cancer
- PMID: 25917569
- PMCID: PMC4476386
- DOI: 10.1038/ncomms7885
Galanin modulates the neural niche to favour perineural invasion in head and neck cancer
Abstract
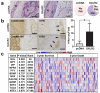
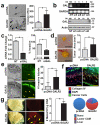
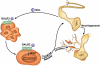
Perineural invasion (PNI) is an indicator of poor survival in multiple cancers. Unfortunately, there is no targeted treatment for PNI since the molecular mechanisms are largely unknown. PNI is an active process, suggesting that cancer cells communicate with nerves. However, nerve-tumour crosstalk is understudied due to the lack of in vivo models to investigate the mechanisms. Here we developed an in vivo model of PNI to characterize this interaction. We show that the neuropeptide galanin (GAL) initiates nerve-tumour crosstalk via activation of its G protein-coupled receptor, GALR2. Our data reveal a novel mechanism by which GAL from nerves stimulates GALR2 on cancer cells to induce NFATC2-mediated transcription of cyclooxygenase-2 and GAL. Prostaglandin E2 promotes cancer invasion, and in a feedback mechanism, GAL released by cancer induces neuritogenesis, facilitating PNI. This study describes a novel in vivo model for PNI and reveals the dynamic interaction between nerve and cancer.
Figures




References
-
- Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. - PubMed
-
- Binmadi NO, Basile JR. Perineural invasion in oral squamous cell carcinoma: a discussion of significance and review of the literature. Oral Oncol. 2011;47:1005–1010. - PubMed
-
- Ayala GE, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. - PubMed
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. - PubMed
-
- Johnston M, Yu E, Kim J. Perineural invasion and spread in head and neck cancer. Expert Rev Anticancer Ther. 2012;12:359–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC000456/DC/NIDCD NIH HHS/United States
- DC000456/DC/NIDCD NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- DE022567/DE/NIDCR NIH HHS/United States
- K02 DE019513/DE/NIDCR NIH HHS/United States
- DE021293/DE/NIDCR NIH HHS/United States
- DE018512/DE/NIDCR NIH HHS/United States
- R01 DE018512/DE/NIDCR NIH HHS/United States
- DE019513/DE/NIDCR NIH HHS/United States
- DE024384/DE/NIDCR NIH HHS/United States
- R21 DE024384/DE/NIDCR NIH HHS/United States
- F30 DE021293/DE/NIDCR NIH HHS/United States
- DC009982/DC/NIDCD NIH HHS/United States
- R01 DE022567/DE/NIDCR NIH HHS/United States
- F32 DC000456/DC/NIDCD NIH HHS/United States
- R01 DC009982/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

