Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan
- PMID: 25917673
- PMCID: PMC4447616
- DOI: 10.1016/j.vaccine.2015.04.055
Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan
Abstract
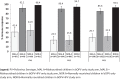
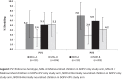
Reaching high population immunity against polioviruses (PV) is essential to achieving global polio eradication. Efficacy of oral poliovirus vaccine (OPV) varies and is lower among children living in tropical areas with impoverished environments. Malnutrition found as a risk factor for lower serological protection against PV. We compared whether inactivated polio vaccine (IPV) can be used to rapidly close the immunity gap among chronically malnourished (stunted) infants in Pakistan who will not be eligible for the 14 week IPV dose in routine EPI schedule. A phase 3, multicenter 4-arm randomized controlled trial conducted at five Primary Health Care (PHC) centers in Karachi, Pakistan. Infants, 9-12 months were stratified by length for age Z score into chronically malnourished and normally nourished. Infants were randomized to receive one dose of either bivalent OPV (bOPV) alone or bOPV+IPV. Baseline seroprevalence of PV antibodies and serum immune response to study vaccine dose were assessed by neutralization assay. Vaccine PV shedding in stool was evaluated 7 days after a bOPV challenge dose. Sera and stool were analyzed from 852/928 (92%) enrolled children. At baseline, the seroprevalence was 85.6% (n=386), 73.6% (n=332), and 70.7% (n=319) in malnourished children against PV types 1, 2 and 3 respectively; and 94.1% (n=448), 87.0% (n=441) and 83.6% (n=397) in the normally nourished group (p<0.05). Children had previously received 9-10 doses of bOPV (80%) or tOPV (20%). One dose of IPV+bOPV given to malnourished children increased their serological protection (PV1, n=201, 97.6%; PV2, n=198, 96.1% and PV3, n=189, 91.7%) to parity with normally nourished children who had not received IPV (p=<0.001). Seroconversion and boosting for all three serotypes was significantly more frequent in children who received IPV+bOPV than in those with bOPV only (p<0.001) in both strata. Shedding of polioviruses in stool did not differ between study groups and ranged from 2.4% (n=5) to 7.1% (n=15). In malnourished children the shedding was reduced after bOPV+IPV compared to bOPV only. Chronically malnourished infants were more likely to be unprotected against polioviruses than normal infants. bOPV+IPV helped close the immunity gap better than bOPV alone.
Keywords: Chronic malnutrition; Polio vaccine immunity; Polio vaccine trial; Seroconversion.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- World Health Organization; 2014. Cases of wild poliovirus by country and year. [updated 2014; cited 08/08/2014]; Available from: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli....
-
- Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission – worldwide, January 2011–March 2012. Morb Mortal Wkly Rep. 2012;61(May (19)):353–357. - PubMed
-
- Sutter R.W., Kew O.M., Cochi S.L., Aylward R.B. Poliovirus vaccine—live. In: Plotkin S.A., Orenstein W.A., Offit P.A., editors. Vaccines. 6th ed. W.B. Saunders; London: 2013. pp. 598–645. [chapter 28]
-
- Caceres V.M., Sutter R.W. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis. 2001;33(August (4)):531–541. [Historical article. Research support, U.S. Gov’t, P.H.S. Review] - PubMed
-
- Hamer D. 1st ed. Elsevier; 2010. Poliomyelitis. Public health and infectious diseases.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

