Functional analysis of naturally occurring DCLRE1C mutations and correlation with the clinical phenotype of ARTEMIS deficiency
- PMID: 25917813
- PMCID: PMC4494888
- DOI: 10.1016/j.jaci.2015.03.005
Functional analysis of naturally occurring DCLRE1C mutations and correlation with the clinical phenotype of ARTEMIS deficiency
Abstract
Background: The endonuclease ARTEMIS, which is encoded by the DCLRE1C gene, is a component of the nonhomologous end-joining pathway and participates in hairpin opening during the V(D)J recombination process and repair of a subset of DNA double-strand breaks. Patients with ARTEMIS deficiency usually present with severe combined immunodeficiency (SCID) and cellular radiosensitivity, but hypomorphic mutations can cause milder phenotypes (leaky SCID).
Objective: We sought to correlate the functional effect of human DCLRE1C mutations on phenotypic presentation in patients with ARTEMIS deficiency.
Methods: We studied the recombination and DNA repair activity of 41 human DCLRE1C mutations in Dclre1c(-/-) v-abl kinase-transformed pro-B cells retrovirally engineered with a construct that allows quantification of recombination activity by means of flow cytometry. For assessment of DNA repair efficacy, resolution of γH2AX accumulation was studied after ionizing radiation.
Results: Low or absent activity was detected for mutations causing a typical SCID phenotype. Most of the patients with leaky SCID were compound heterozygous for 1 loss-of-function and 1 hypomorphic allele, with significant residual levels of recombination and DNA repair activity. Deletions disrupting the C-terminus result in truncated but partially functional proteins and are often associated with leaky SCID. Overexpression of hypomorphic mutants might improve the functional defect.
Conclusions: Correlation between the nature and location of DCLRE1C mutations, functional activity, and the clinical phenotype has been observed. Hypomorphic variants that have been reported in the general population can be disease causing if combined in trans with a loss-of-function allele. Therapeutic strategies aimed at inducing overexpression of hypomorphic alleles might be beneficial.
Keywords: ARTEMIS deficiency; DCLRE1C mutations; DNA repair; V(D)J recombination; nonhomologous end-joining; severe combined immunodeficiency.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

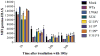

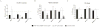
References
-
- Le Deist F, Poinsignon C, Moshous D, Fischer A, de Villartay JP. Artemis sheds new light on V(D)J recombination. Immunol Rev. 2004;200:142–55. - PubMed
-
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. - PubMed
-
- Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–94. - PubMed
-
- van der Burg M, Verkaik NS, den Dekker AT, Barendregt BH, Pico-Knijnenburg I, Tezcan I, et al. Defective Artemis nuclease is characterized by coding joints with microhomology in long palindromic-nucleotide stretches. Eur J Immunol. 2007;37:3522–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

