Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease
- PMID: 25918124
- PMCID: PMC4457157
- DOI: 10.1242/jcs.162891
Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease
Abstract
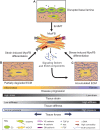
Fibrotic cardiac disease, a leading cause of death worldwide, manifests as substantial loss of function following maladaptive tissue remodeling. Fibrosis can affect both the heart valves and the myocardium and is characterized by the activation of fibroblasts and accumulation of extracellular matrix. Valvular interstitial cells and cardiac fibroblasts, the cell types responsible for maintenance of cardiac extracellular matrix, are sensitive to changing mechanical environments, and their ability to sense and respond to mechanical forces determines both normal development and the progression of disease. Recent studies have uncovered specific adhesion proteins and mechano-sensitive signaling pathways that contribute to the progression of fibrosis. Integrins form adhesions with the extracellular matrix, and respond to changes in substrate stiffness and extracellular matrix composition. Cadherins mechanically link neighboring cells and are likely to contribute to fibrotic disease propagation. Finally, transition to the active myofibroblast phenotype leads to maladaptive tissue remodeling and enhanced mechanotransductive signaling, forming a positive feedback loop that contributes to heart failure. This Commentary summarizes recent findings on the role of mechanotransduction through integrins and cadherins to perpetuate mechanically induced differentiation and fibrosis in the context of cardiac disease.
Keywords: Cadherin; Integrin; Myofibroblast.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Afek A., Shoenfeld Y., Manor R., Goldberg I., Ziporen L., George J., Polak-Charcon S., Amigo M. C., Garcia-Torres R., Segal R. et al. (1999). Increased endothelial cell expression of alpha3beta1 integrin in cardiac valvulopathy in the primary (Hughes) and secondary antiphospholipid syndrome. Lupus 8, 502-507. 10.1191/096120399678840873 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

