Role of Apolipoprotein E in β-Amyloidogenesis: ISOFORM-SPECIFIC EFFECTS ON PROTOFIBRIL TO FIBRIL CONVERSION OF Aβ IN VITRO AND BRAIN Aβ DEPOSITION IN VIVO
- PMID: 25918154
- PMCID: PMC4463458
- DOI: 10.1074/jbc.M114.622209
Role of Apolipoprotein E in β-Amyloidogenesis: ISOFORM-SPECIFIC EFFECTS ON PROTOFIBRIL TO FIBRIL CONVERSION OF Aβ IN VITRO AND BRAIN Aβ DEPOSITION IN VIVO
Erratum in
-
Role of apolipoprotein E in β-amyloidogenesis: Isoform-specific effects on protofibril to fibril conversion of Aβ in vitro and brain Aβ deposition in vivo.J Biol Chem. 2018 May 11;293(19):7267. doi: 10.1074/jbc.AAC118.003576. J Biol Chem. 2018. PMID: 29752420 Free PMC article. No abstract available.
Abstract
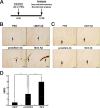
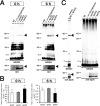
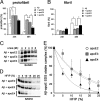
Human APOE ϵ4 allele is a strong genetic risk factor of Alzheimer disease. Neuropathological and genetic studies suggested that apolipoprotein E4 (apoE4) protein facilitates deposition of amyloid β peptide (Aβ) in the brain, although the mechanism whereby apoE4 increases amyloid aggregates remains elusive. Here we show that injection of Aβ protofibrils induced Aβ deposition in the brain of APP transgenic mice, suggesting that Aβ protofibrils acted as a seed for aggregation and deposition of Aβ in vivo. Injection of Aβ protofibrils together with apoE3 significantly attenuated Aβ deposition, whereas apoE4 did not have this effect. In vitro assays revealed that the conversion of Aβ protofibrils to fibrils progressed more slowly upon coincubation with apoE2 or apoE3 compared with that with apoE4. Aβ protofibrils complexed with apoE4 were less stable than those with apoE2 or apoE3. These data suggest that the suppression effect of apoE2 or apoE3 on the structural conversion of Aβ protofibrils to fibrils is stronger than those of apoE4, thereby impeding β-amyloid deposition.
Keywords: Alzheimer disease; amyloid; amyloid-β; apolipoprotein E; protein stability.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Selkoe D. J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 - PubMed
-
- Dickson D. W. (1997) The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 56, 321–339 - PubMed
-
- Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. (1987) Astrocyte synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 917, 148–161 - PubMed
-
- Nakai M., Kawamata T., Taniguchi T., Maeda K., Tanaka C. (1996) Expression of apolipoprotein E mRNA in rat microglia. Neurosci. Lett. 211, 41–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

