Symptoms and Distress in Children With Advanced Cancer: Prospective Patient-Reported Outcomes From the PediQUEST Study
- PMID: 25918277
- PMCID: PMC4451175
- DOI: 10.1200/JCO.2014.59.1222
Symptoms and Distress in Children With Advanced Cancer: Prospective Patient-Reported Outcomes From the PediQUEST Study
Abstract
Purpose: Thousands of children are living with advanced cancer; yet patient-reported outcomes (PROs) have rarely been used to describe their experiences. We aimed to describe symptom distress in 104 children age 2 years or older with advanced cancer enrolled onto the Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) Study (multisite clinical trial evaluating an electronic PRO system).
Methods: Symptom data were collected using age- and respondent-adapted versions of the PediQUEST Memorial Symptom Assessment Scale (PQ-MSAS) at most once per week. Clinical and treatment data were obtained from medical records. Individual symptom scores were dichotomized into high/low distress. Determinants of PQ-MSAS scores were explored using linear mixed-effects models.
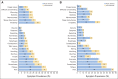
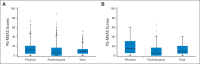
Results: During 9 months of follow-up, PQ-MSAS was administered 920 times: 459 times in teens (99% self-report), 249 times in children ages 7 to 12 years (96% child/parent report), and 212 times in those ages 2 to 6 years (parent reports). Common symptoms included pain (48%), fatigue (46%), drowsiness (39%), and irritability (37%); most scores indicated high distress. Among the 73 PQ-MSAS surveys administered in the last 12 weeks of life, pain was highly prevalent (62%; 58% with high distress). Being female, having a brain tumor, experiencing recent disease progression, and receiving moderate- or high-intensity cancer-directed therapy in the prior 10 days were associated with worse PQ-MSAS scores. In the final 12 weeks of life, receiving mild cancer-directed therapy was associated with improved psychological PQ-MSAS scores.
Conclusion: Children with advanced cancer experience high symptom distress. Strategies to promote intensive symptom management are indicated, especially with disease progression or administration of intensive treatments.
Trial registration: ClinicalTrials.gov NCT01838564.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- American Cancer Society. Cancer facts and figures 2012. Atlanta, GA: American Cancer Society; 2012.
-
- Field MJ, Behrman RE. When Children Die: Improving Palliative and End-of-Life Care for Children and Their Families. Washington, DC: National Academies Press; 2003. https://www.iom.edu/Reports/2002/When-Children-Die-Improving-Palliative-.... - PubMed
-
- Heath JA, Clarke NE, Donath SM, et al. Symptoms and suffering at the end of life in children with cancer: An Australian perspective. Med J Aust. 2010;192:71–75. - PubMed
-
- Jalmsell L, Kreicbergs U, Onelöv E, et al. Symptoms affecting children with malignancies during the last month of life: A nationwide follow-up. Pediatrics. 2006;117:1314–1320. - PubMed
-
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342:326–333. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

