Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV
- PMID: 25918346
- PMCID: PMC4574015
- DOI: 10.1182/blood-2015-01-617993
Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV
Abstract
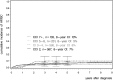
We studied the influence of comorbidities on remission rate and overall survival (OS) in patients with chronic myeloid leukemia (CML). Participants of the CML Study IV, a randomized 5-arm trial designed to optimize imatinib therapy, were analyzed for comorbidities at diagnosis using the Charlson Comorbidity Index (CCI); 511 indexed comorbidities were reported in 1519 CML patients. Age was an additional risk factor in 863 patients. Resulting CCI scores were as follows: CCI 2, n = 589; CCI 3 or 4, n = 599; CCI 5 or 6, n = 229; and CCI ≥ 7, n = 102. No differences in cumulative incidences of accelerated phase, blast crisis, or remission rates were observed between patients in the different CCI groups. Higher CCI was significantly associated with lower OS probabilities. The 8-year OS probabilities were 93.6%, 89.4%, 77.6%, and 46.4% for patients with CCI 2, 3 to 4, 5 to 6, and ≥7, respectively. In multivariate analysis, CCI was the most powerful predictor of OS, which was still valid after removal of its age-related components. Comorbidities have no impact on treatment success but do have a negative effect on OS, indicating that survival of patients with CML is determined more by comorbidities than by CML itself. OS may therefore be inappropriate as an outcome measure for specific CML treatments. The trial was registered at www.clinicaltrials.gov as #NCT00055874.
© 2015 by The American Society of Hematology.
Figures



Comment in
-
Living with CML: is death no longer the end (point)?Blood. 2015 Jul 2;126(1):2-4. doi: 10.1182/blood-2015-05-644732. Blood. 2015. PMID: 26138535 No abstract available.
-
Molecular medicine: Precision oncology is not an illusion.Nature. 2016 Nov 17;539(7629):357. doi: 10.1038/539357e. Nature. 2016. PMID: 27853199 No abstract available.
References
-
- Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-α in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29(12):1634–1642. - PubMed
-
- Hehlmann R, Müller MC, Lauseker M, et al. Deep Molecular Response (MR4.5) is reached by the majority of imatinib-treated patients, predicts survival, and is achieved faster by optimized high-dose imatinib - results from the randomized CML-Study IV. J Clin Oncol. 2014;32(5):415–423. - PubMed
-
- Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. - PubMed
-
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

