Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist
- PMID: 25918380
- PMCID: PMC4434738
- DOI: 10.1073/pnas.1421785112
Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist
Erratum in
-
Correction for Stoveken et al., Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist.Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):E3452. doi: 10.1073/pnas.1510107112. Epub 2015 Jun 9. Proc Natl Acad Sci U S A. 2015. PMID: 26060312 Free PMC article. No abstract available.
Abstract
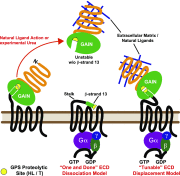
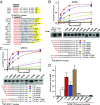
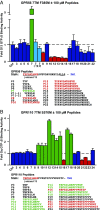
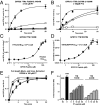
The large class of adhesion G protein-coupled receptors (aGPCRs) bind extracellular matrix or neighboring cell-surface ligands to regulate organ and tissue development through an unknown activation mechanism. We examined aGPCR activation using two prototypical aGPCRs, GPR56 and GPR110. Active dissociation of the noncovalently bound GPR56 or GPR110 extracellular domains (ECDs) from the respective seven-transmembrane (7TM) domains relieved an inhibitory influence and permitted both receptors to activate defined G protein subtypes. After ECD displacement, the newly revealed short N-terminal stalk regions of the 7TM domains were found to be essential for G protein activation. Synthetic peptides comprising these stalks potently activated GPR56 or GPR110 in vitro or in cells, demonstrating that the stalks comprise a tethered agonist that was encrypted within the ECD. Establishment of an aGPCR activation mechanism provides a rational platform for the development of aGPCR synthetic modulators that could find clinical utility toward aGPCR-directed disease.
Keywords: G proteins; GPR110; GPR56; adhesion GPCRs; tethered agonist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bjarnadóttir TK, et al. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84(1):23–33. - PubMed
-
- Langenhan T, Aust G, Hamann J. Sticky signaling—adhesion class G protein-coupled receptors take the stage. Sci Signal. 2013;6(276):re3. - PubMed
-
- Aust G. Adhesion-GPCRs in tumorigenesis. Adv Exp Med Biol. 2010;706:109–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

