Evasion of early antiviral responses by herpes simplex viruses
- PMID: 25918478
- PMCID: PMC4396904
- DOI: 10.1155/2015/593757
Evasion of early antiviral responses by herpes simplex viruses
Abstract
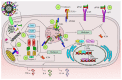
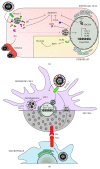
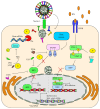
Besides overcoming physical constraints, such as extreme temperatures, reduced humidity, elevated pressure, and natural predators, human pathogens further need to overcome an arsenal of antimicrobial components evolved by the host to limit infection, replication and optimally, reinfection. Herpes simplex virus-1 (HSV-1) and herpes simplex virus-2 (HSV-2) infect humans at a high frequency and persist within the host for life by establishing latency in neurons. To gain access to these cells, herpes simplex viruses (HSVs) must replicate and block immediate host antiviral responses elicited by epithelial cells and innate immune components early after infection. During these processes, infected and noninfected neighboring cells, as well as tissue-resident and patrolling immune cells, will sense viral components and cell-associated danger signals and secrete soluble mediators. While type-I interferons aim at limiting virus spread, cytokines and chemokines will modulate resident and incoming immune cells. In this paper, we discuss recent findings relative to the early steps taking place during HSV infection and replication. Further, we discuss how HSVs evade detection by host cells and the molecular mechanisms evolved by these viruses to circumvent early antiviral mechanisms, ultimately leading to neuron infection and the establishment of latency.
Figures
Similar articles
-
Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):599-607. doi: 10.1089/jir.2009.0074. J Interferon Cytokine Res. 2009. PMID: 19694546 Review.
-
Herpes Simplex Virus Type 1 Interactions with the Interferon System.Int J Mol Sci. 2020 Jul 21;21(14):5150. doi: 10.3390/ijms21145150. Int J Mol Sci. 2020. PMID: 32708188 Free PMC article. Review.
-
Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms.Front Immunol. 2021 May 31;12:644664. doi: 10.3389/fimmu.2021.644664. eCollection 2021. Front Immunol. 2021. PMID: 34135889 Free PMC article. Review.
-
Evasion of host antiviral innate immunity by HSV-1, an update.Virol J. 2016 Mar 8;13:38. doi: 10.1186/s12985-016-0495-5. Virol J. 2016. PMID: 26952111 Free PMC article. Review.
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
Cited by
-
Oncolytic herpes simplex virus interactions with the host immune system.Curr Opin Virol. 2016 Dec;21:26-34. doi: 10.1016/j.coviro.2016.07.007. Epub 2016 Aug 3. Curr Opin Virol. 2016. PMID: 27497296 Free PMC article. Review.
-
Nrf2 Negatively Regulates Type I Interferon Responses and Increases Susceptibility to Herpes Genital Infection in Mice.Front Immunol. 2019 Sep 6;10:2101. doi: 10.3389/fimmu.2019.02101. eCollection 2019. Front Immunol. 2019. PMID: 31555293 Free PMC article.
-
Oncolytic herpes simplex virus and immunotherapy.BMC Immunol. 2018 Dec 18;19(1):40. doi: 10.1186/s12865-018-0281-9. BMC Immunol. 2018. PMID: 30563466 Free PMC article. Review.
-
Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection.Antioxidants (Basel). 2023 May 29;12(6):1170. doi: 10.3390/antiox12061170. Antioxidants (Basel). 2023. PMID: 37371900 Free PMC article.
-
Blue light irradiation exerts anti-viral and anti-inflammatory properties against herpes simplex virus type 1 infection.J Photochem Photobiol B. 2023 Feb;239:112632. doi: 10.1016/j.jphotobiol.2022.112632. Epub 2022 Dec 22. J Photochem Photobiol B. 2023. PMID: 36608399 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 Years—United States, 2005–2008. Morbidity and Mortality Weekly Report. 2010;59(15):456–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

