Cervical Lymph Nodes as a Selective Niche for Brucella during Oral Infections
- PMID: 25919005
- PMCID: PMC4412401
- DOI: 10.1371/journal.pone.0121790
Cervical Lymph Nodes as a Selective Niche for Brucella during Oral Infections
Abstract
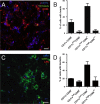
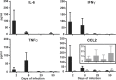
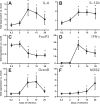
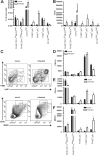
Cervical lymph nodes (CLN) are the first lymph nodes encountered by material taking the oral route. To study their role in orally acquired infections, we analyzed 307 patients of up to 14 years treated in the university clinic of Skopje, Macedonia, for brucellosis, a zoonotic bacterial disease frequently acquired by ingestion of contaminated dairy products. From these children, 36% had lymphadenopathy. Among orally infected children, lymphadenopathy with CLN being the only lymph nodes affected was significantly more frequent as compared to those infected by contact with animals (83% vs. 63%), suggesting a possible involvement of CLN during orally acquired human brucellosis. Using a murine model where bacteria are delivered into the oral cavity, we show that Brucella quickly and selectively colonize the CLN where they proliferate and persist over long periods of time for up to 50 days post-infection. A similar efficient though less specific drainage to CLN was found for Brucella, Salmonella typhimurium and fluorescent microspheres delivered by gavage, a pathway likely representing a mixed infection mode of intragastric and oral infection, suggesting a central pathway of drained material. Microspheres as well as bacteria drained to CLN predominately reside in cells expressing CD68 and no or low levels of CD11c. Even though no systemic response could be detected, Brucella induced a locally restricted inflammatory reaction with increased expression levels of interferon γ, interleukin (IL)-6, IL-12, granzyme B and a delayed induction of Nos2. Inflammation led to pronounced lymphadenopathy, infiltration of macrophages/monocytes expressing high levels of major histocompatibility complex II and to formation of epitheloid granulomas. Together, these results highlight the role of CLN in oral infections as both, an initial and efficient trap for bacterial invaders and as possible reservoir for chronic pathogens. They likewise cast a new light on the significance of oral routes for means of vaccination.
Conflict of interest statement
Figures





References
-
- Dasaraju PV, Liu C. Infections of the Respiratory System. In: Baron S, editor. Medical Microbiology. 4th ed. Galveston (TX); 1996. - PubMed
-
- Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy 2007;92: 58–70. - PubMed
-
- Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 1993;19: 480–488. - PubMed
-
- Wolvers DA, Coenen-de Roo CJ, Mebius RE, van der Cammen MJ, Tirion F, et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol 1999;162: 1994–1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

