Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial
- PMID: 25919061
- PMCID: PMC5029775
- DOI: 10.1002/ejhf.266
Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial
Abstract
Aims: Thrombin is a critical element of crosstalk between pathways contributing to worsening of established heart failure (HF). The aim of this study is to explore the efficacy and safety of rivaroxaban 2.5 mg bid compared with placebo (with standard care) after an exacerbation of HF in patients with reduced ejection fraction (HF-rEF) and documented coronary artery disease.
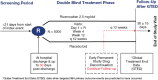
Methods: This is an international prospective, multicentre, randomized, double-blind, placebo-controlled, event-driven study of approximately 5000 patients for a targeted 984 events. Patients must have a recent symptomatic exacerbation of HF, increased plasma concentrations of natriuretic peptides (B-type natriuretic peptide ≥200 pg/mL or N-terminal pro-B-type natriuretic peptide ≥800 pg/mL), with left ventricular ejection fraction ≤40% and coronary artery disease. Patients requiring anticoagulation for atrial fibrillation or other conditions will be excluded. After an index event (overnight hospitalization, emergency department or observation unit admission, or unscheduled outpatient parenteral treatment for worsening HF), patients will be randomized 1:1 to rivaroxaban or placebo (with standard of care). The primary efficacy outcome event is a composite of all-cause mortality, myocardial infarction or stroke. The principal safety outcome events are the composite of fatal bleeding or bleeding into a critical space with potential permanent disability, bleeding events requiring hospitalization and major bleeding events according to International Society on Thrombosis and Haemostasis bleeding criteria.
Conclusion: COMMANDER HF is the first prospective study of a target-specific oral antithrombotic agent in HF. It will provide important information regarding rivaroxaban use following an HF event in an HF-rEF patient population with coronary artery disease.
Keywords: antithrombotic; coronary artery disease; heart failure; rivaroxaban; thrombin.
© 2015 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
Comment in
-
Disrupting Virchow's triad: can factor X inhibition reduce risk of adverse outcomes in patients with ischaemic cardiomyopathy?Eur J Heart Fail. 2015 Jul;17(7):647-51. doi: 10.1002/ejhf.296. Epub 2015 May 27. Eur J Heart Fail. 2015. PMID: 26018996 No abstract available.
References
-
- Francis GS, Cogswell R, Thenappan T. The heterogeneity of heart failure: will enhanced phenotyping be necessary for future clinical trial success? J Am Coll Cardiol 2014;64 : 1775–1776. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128 : e240–e327. - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33 : 1787–1847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous