Recent developments in the Suzuki-Miyaura reaction: 2010-2014
- PMID: 25919276
- PMCID: PMC6272665
- DOI: 10.3390/molecules20057528
Recent developments in the Suzuki-Miyaura reaction: 2010-2014
Abstract
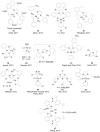
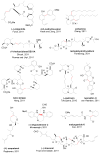
The Suzuki-Miyaura reaction (SMR), involving the coupling of an organoboron reagent and an organic halide or pseudo-halide in the presence of a palladium or nickel catalyst and a base, has arguably become one of most utilized tools for the construction of a C-C bond. This review intends to be general account of all types of catalytic systems, new coupling partners and applications, including the literature between September 2010 and December 2014.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- Miyaura N., Yamada K., Suzuki A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979;20:3437–3440. doi: 10.1016/S0040-4039(01)95429-2. - DOI
-
- Miyaura N. Cross-coupling reactions: A practical guide. Top. Curr. Chem. 2002;219:11–59.
-
- Tsuji J. Palladium Reagents and Catalysis. 2nd ed. Wiley; West Sussex, UK: 2004.
-
- De Meijere A, Diederich F. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Wiley-VCH; Weinheim, Germany: 2004.
-
- Negishi E. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley-Interscience; New York, NY, USA: 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

