Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta-analysis
- PMID: 25919293
- PMCID: PMC4412636
- DOI: 10.1371/journal.pone.0125879
Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta-analysis
Abstract
Introduction: When first line therapy with metformin is insufficient for patients with type 2 diabetes (T2D), the optimal adjunctive therapy is unclear. We assessed the efficacy and safety of adjunctive antidiabetic agents in patients with inadequately controlled T2D on metformin alone.
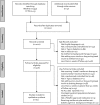
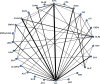
Materials and methods: A search of MEDLINE and CENTRAL, clinicaltrials.gov, regulatory websites was performed. We included randomized controlled trials of 3-12 months duration, evaluating Food and Drug Administration or European Union approved agents (noninsulin and long acting, once daily basal insulins) in patients experiencing inadequate glycemic control with metformin monotherapy (≥ 1500 mg daily or maximally tolerated dose for ≥ 4 weeks). Random-effects network meta-analyses were used to compare the weighted mean difference for changes from baseline in HbA1c, body weight (BW) and systolic blood pressure (SBP), and the risk of developing hypoglycemia, urinary (UTI) and genital tract infection (GTI).
Results: Sixty-two trials evaluating 25 agents were included. All agents significantly reduced HbA1c vs. placebo; albeit not to the same extent (range, 0.43% for miglitol to 1.29% for glibenclamide). Glargine, sulfonylureas (SUs) and nateglinide were associated with increased hypoglycemia risk vs. placebo (range, 4.00-11.67). Sodium glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 analogs, miglitol and empagliflozin/linagliptin significantly reduced BW (range, 1.15-2.26 kg) whereas SUs, thiazolindinediones, glargine and alogliptin/pioglitazone caused weight gain (range, 1.19-2.44 kg). SGLT2 inhibitors, empagliflozin/linagliptin, liraglutide and sitagliptin decreased SBP (range, 1.88-5.43 mmHg). No therapy increased UTI risk vs. placebo; however, SGLT2 inhibitors were associated with an increased risk of GTI (range, 2.16-8.03).
Conclusions: Adding different AHAs to metformin was associated with varying effects on HbA1c, BW, SBP, hypoglycemia, UTI and GTI which should impact clinician choice when selecting adjunctive therapy.
Conflict of interest statement
Figures
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. 10.2337/dc12-0413 - DOI - PMC - PubMed
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. (Last accessed on June 27, 2014).
-
- Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–37. 10.1016/j.jval.2011.01.011 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

