Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers
- PMID: 25919418
- PMCID: PMC4492699
- DOI: 10.1002/anie.201500799
Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers
Abstract
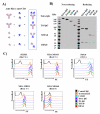
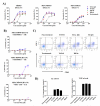
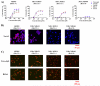
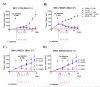
Four different formats of bispecific antibodies (bsAbs) were generated that consist of anti-Her2 IgG or Fab site-specifically conjugated to anti-CD3 Fab using the genetically encoded noncanonical amino acid. These bsAbs varied in valency or in the presence or absence of an Fc domain. Different valencies did not significantly affect antitumor efficacy, whereas the presence of an Fc domain enhanced cytotoxic activity, but triggered antigen-independent T-cell activation. We show that the bsAbs can efficiently redirect T cells to kill all Her2 expressing cancer cells, including Her2 1+ cancers, both in vitro and in rodent xenograft models. This work increases our understanding of the structural features that affect bsAb activity, and underscores the potential of bsAbs as a promising therapeutic option for breast cancer patients with low or heterogeneous Her2 expression.
Keywords: T-cell activation; antibody drug conjugates; bispecific antibodies; breast cancers; noncanonical amino acids.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. J.Clin.Oncol. 2002;20:719–726. - PubMed
-
- Krop I, Winer EP. Clin.Cancer Res. 2014;20:15–20. - PubMed
-
- Keating GM. Drugs. 2012;72:353–360. - PubMed
-
- Owens MA, Horten BC, Da Silva MM. Clin.Breast Cancer. 2004;5:63–69. - PubMed
-
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Cancer Res. 2008;68:9280–9290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

