Economic evaluation of DNA ploidy analysis vs liquid-based cytology for cervical screening
- PMID: 25919612
- PMCID: PMC4580387
- DOI: 10.1038/bjc.2015.95
Economic evaluation of DNA ploidy analysis vs liquid-based cytology for cervical screening
Abstract
Background: DNA ploidy analysis involves automated quantification of chromosomal aneuploidy, a potential marker of progression toward cervical carcinoma. We evaluated the cost-effectiveness of this method for cervical screening, comparing five ploidy strategies (using different numbers of aneuploid cells as cut points) with liquid-based Papanicolaou smear and no screening.
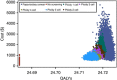
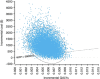
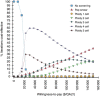
Methods: A state-transition Markov model simulated the natural history of HPV infection and possible progression into cervical neoplasia in a cohort of 12-year-old females. The analysis evaluated cost in 2012 US$ and effectiveness in quality-adjusted life-years (QALYs) from a health-system perspective throughout a lifetime horizon in the US setting. We calculated incremental cost-effectiveness ratios (ICERs) to determine the best strategy. The robustness of optimal choices was examined in deterministic and probabilistic sensitivity analyses.
Results: In the base-case analysis, the ploidy 4 cell strategy was cost-effective, yielding an increase of 0.032 QALY and an ICER of $18 264/QALY compared to no screening. For most scenarios in the deterministic sensitivity analysis, the ploidy 4 cell strategy was the only cost-effective strategy. Cost-effectiveness acceptability curves showed that this strategy was more likely to be cost-effective than the Papanicolaou smear.
Conclusion: Compared to the liquid-based Papanicolaou smear, screening with a DNA ploidy strategy appeared less costly and comparably effective.
Figures
References
-
- Agency for Healthcare Research and Quality 2012. Screening for Cervical Cancer: Clinical Summary of U.S. Preventive Services Task Force Recommendation. AHRQ Publication No. 11-05156-EF-3, March 2012.
-
- Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22 (12:2675–2686. - PubMed
-
- Bergeron C, Largeron N, McAllister R, Mathevet P, Remy V. Cost-effectiveness analysis of the introduction of a quadrivalent human papillomavirus vaccine in France. Int J Technol Assess Health Care. 2008;24 (1:10–19. - PubMed
-
- Bocking A, Nguyen VQ. Diagnostic and prognostic use of DNA image cytometry in cervical squamous intraepithelial lesions and invasive carcinoma. Cancer. 2004;102 (1:41–54. - PubMed
-
- Cantor SB, Cardenas-Turanzas M, Cox DD, Atkinson EN, Nogueras-Gonzalez GM, Beck JR, Follen M, Benedet JL. Accuracy of colposcopy in the diagnostic setting compared with the screening setting. Obstet Gynecol. 2008;111 (1:7–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

