A Complete Pathway Model for Lipid A Biosynthesis in Escherichia coli
- PMID: 25919634
- PMCID: PMC4412817
- DOI: 10.1371/journal.pone.0121216
A Complete Pathway Model for Lipid A Biosynthesis in Escherichia coli
Abstract
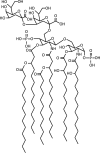
Lipid A is a highly conserved component of lipopolysaccharide (LPS), itself a major component of the outer membrane of Gram-negative bacteria. Lipid A is essential to cells and elicits a strong immune response from humans and other animals. We developed a quantitative model of the nine enzyme-catalyzed steps of Escherichia coli lipid A biosynthesis, drawing parameters from the experimental literature. This model accounts for biosynthesis regulation, which occurs through regulated degradation of the LpxC and WaaA (also called KdtA) enzymes. The LpxC degradation signal appears to arise from the lipid A disaccharide concentration, which we deduced from prior results, model results, and new LpxK overexpression results. The model agrees reasonably well with many experimental findings, including the lipid A production rate, the behaviors of mutants with defective LpxA enzymes, correlations between LpxC half-lives and cell generation times, and the effects of LpxK overexpression on LpxC concentrations. Its predictions also differ from some experimental results, which suggest modifications to the current understanding of the lipid A pathway, such as the possibility that LpxD can replace LpxA and that there may be metabolic channeling between LpxH and LpxB. The model shows that WaaA regulation may serve to regulate the lipid A production rate when the 3-deoxy-D-manno-oct-2-ulosonic acid (KDO) concentration is low and/or to control the number of KDO residues that get attached to lipid A. Computation of flux control coefficients showed that LpxC is the rate-limiting enzyme if pathway regulation is ignored, but that LpxK is the rate-limiting enzyme if pathway regulation is present, as it is in real cells. Control also shifts to other enzymes if the pathway substrate concentrations are not in excess. Based on these results, we suggest that LpxK may be a much better drug target than LpxC, which has been pursued most often.
Conflict of interest statement
Figures



Similar articles
-
Crosstalk between the lipopolysaccharide and phospholipid pathways during outer membrane biogenesis in Escherichia coli.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):3108-13. doi: 10.1073/pnas.1521168113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929331 Free PMC article.
-
yciM is an essential gene required for regulation of lipopolysaccharide synthesis in Escherichia coli.Mol Microbiol. 2014 Jan;91(1):145-57. doi: 10.1111/mmi.12452. Epub 2013 Nov 24. Mol Microbiol. 2014. PMID: 24266962
-
Pathway for lipid A biosynthesis in Arabidopsis thaliana resembling that of Escherichia coli.Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11387-92. doi: 10.1073/pnas.1108840108. Epub 2011 Jun 27. Proc Natl Acad Sci U S A. 2011. PMID: 21709257 Free PMC article.
-
Antibacterial Drug Discovery Targeting the Lipopolysaccharide Biosynthetic Enzyme LpxC.Cold Spring Harb Perspect Med. 2016 Jul 1;6(7):a025304. doi: 10.1101/cshperspect.a025304. Cold Spring Harb Perspect Med. 2016. PMID: 27235477 Free PMC article. Review.
-
Structure- and Ligand-Dynamics-Based Design of Novel Antibiotics Targeting Lipid A Enzymes LpxC and LpxH in Gram-Negative Bacteria.Acc Chem Res. 2021 Apr 6;54(7):1623-1634. doi: 10.1021/acs.accounts.0c00880. Epub 2021 Mar 15. Acc Chem Res. 2021. PMID: 33720682 Free PMC article. Review.
Cited by
-
A kinetic rationale for functional redundancy in fatty acid biosynthesis.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23557-23564. doi: 10.1073/pnas.2013924117. Epub 2020 Sep 3. Proc Natl Acad Sci U S A. 2020. PMID: 32883882 Free PMC article.
-
Alterations of the intestinal microbiota in age-related macular degeneration.Front Microbiol. 2023 Apr 5;14:1069325. doi: 10.3389/fmicb.2023.1069325. eCollection 2023. Front Microbiol. 2023. PMID: 37089564 Free PMC article.
-
Identification of the Shigella flexneri Wzy Domain Modulating WzzpHS-2 Interaction and Detection of the Wzy/Wzz/Oag Complex.J Bacteriol. 2022 Sep 20;204(9):e0022422. doi: 10.1128/jb.00224-22. Epub 2022 Aug 18. J Bacteriol. 2022. PMID: 35980183 Free PMC article.
-
Essential Genes of Vibrio anguillarum and Other Vibrio spp. Guide the Development of New Drugs and Vaccines.Front Microbiol. 2021 Oct 20;12:755801. doi: 10.3389/fmicb.2021.755801. eCollection 2021. Front Microbiol. 2021. PMID: 34745063 Free PMC article.
-
Degenerative Cervical Myelopathy induces sex-specific dysbiosis in mice.Front Microbiol. 2023 Oct 20;14:1229783. doi: 10.3389/fmicb.2023.1229783. eCollection 2023. Front Microbiol. 2023. PMID: 37928672 Free PMC article.
References
-
- Nikaido H. Outer membrane In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin EC C, Low KB Jr, Magasanik B, et al. editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington DC: American Society for Microbiology; 1996. pp. 29–47.
-
- Walker SL, Redman JA, Elimelech M. Role of Cell Surface Lipopolysaccharides in Escherichia coli K12 adhesion and transport. Langmuir. 2004; 20(18): 7736–7746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

