Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells
- PMID: 25919692
- PMCID: PMC4412670
- DOI: 10.1371/journal.pone.0126686
Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells
Abstract
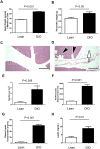
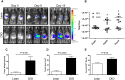
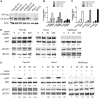
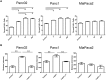
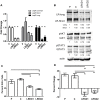
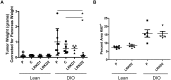
Obesity has been implicated as a significant risk factor for development of pancreatic cancer. In the setting of obesity, a systemic chronic inflammatory response is characterized by alterations in the production and secretion of a wide variety of growth factors. Leptin is a hormone whose level increases drastically in the serum of obese patients. High fat diet induced obesity in mice leads to an overall increased body weight, pancreatic weight, serum leptin, and pancreatic tissue leptin levels. Here we report the contribution of obesity and leptin to pancreatic cancer growth utilizing an in vivo orthotopic murine pancreatic cancer model, which resulted in increased tumor proliferation with concomitant increased tumor burden in the diet induced obese mice compared to lean mice. Human and murine pancreatic cancer cell lines were found to express the short as well as the long form of the leptin receptor and functionally responded to leptin induced activation through an increased phosphorylation of AKT473. In vitro, leptin stimulation increased cellular migration which was blocked by addition of a PI3K inhibitor. In vivo, depletion of the leptin receptor through shRNA knockdown partially abrogated increased orthotopic tumor growth in obese mice. These findings suggest that leptin contributes to pancreatic tumor growth through activation of the PI3K/AKT pathway, which promotes pancreatic tumor cell migration.
Conflict of interest statement
Figures
Similar articles
-
Leptin receptor mediates the proliferation and glucose metabolism of pancreatic cancer cells via AKT pathway activation.Mol Med Rep. 2020 Feb;21(2):945-952. doi: 10.3892/mmr.2019.10855. Epub 2019 Nov 29. Mol Med Rep. 2020. PMID: 31789415
-
c-Jun NH(2)-terminal kinase mediates leptin-stimulated androgen-independent prostate cancer cell proliferation via signal transducer and activator of transcription 3 and Akt.Biochim Biophys Acta. 2008 Oct;1782(10):593-604. doi: 10.1016/j.bbadis.2008.07.005. Epub 2008 Aug 5. Biochim Biophys Acta. 2008. PMID: 18718531
-
Selective Deletion of Leptin Signaling in Endothelial Cells Enhances Neointima Formation and Phenocopies the Vascular Effects of Diet-Induced Obesity in Mice.Arterioscler Thromb Vasc Biol. 2017 Sep;37(9):1683-1697. doi: 10.1161/ATVBAHA.117.309798. Epub 2017 Jul 13. Arterioscler Thromb Vasc Biol. 2017. PMID: 28705795
-
Multifaceted leptin network: the molecular connection between obesity and breast cancer.J Mammary Gland Biol Neoplasia. 2013 Dec;18(3-4):309-20. doi: 10.1007/s10911-013-9308-2. Epub 2013 Nov 10. J Mammary Gland Biol Neoplasia. 2013. PMID: 24214584 Free PMC article. Review.
-
Obesity and respiratory infections: does excess adiposity weigh down host defense?Pulm Pharmacol Ther. 2013 Aug;26(4):412-9. doi: 10.1016/j.pupt.2012.04.006. Epub 2012 May 24. Pulm Pharmacol Ther. 2013. PMID: 22634305 Free PMC article. Review.
Cited by
-
Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer.World J Gastroenterol. 2016 Feb 28;22(8):2441-59. doi: 10.3748/wjg.v22.i8.2441. World J Gastroenterol. 2016. PMID: 26937133 Free PMC article. Review.
-
The Adipokine Component in the Molecular Regulation of Cancer Cell Survival, Proliferation and Metastasis.Pathol Oncol Res. 2021 Sep 13;27:1609828. doi: 10.3389/pore.2021.1609828. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34588926 Free PMC article. Review.
-
A Study on the Immunohistochemical Expressions of Leptin and Leptin Receptor in Clear Cell Renal Cell Carcinoma.Biomed Res Int. 2020 Aug 4;2020:3682086. doi: 10.1155/2020/3682086. eCollection 2020. Biomed Res Int. 2020. PMID: 32802842 Free PMC article.
-
Increased Adiposity Enhances the Accumulation of MDSCs in the Tumor Microenvironment and Adipose Tissue of Pancreatic Tumor-Bearing Mice and in Immune Organs of Tumor-Free Hosts.Nutrients. 2019 Dec 10;11(12):3012. doi: 10.3390/nu11123012. Nutrients. 2019. PMID: 31835454 Free PMC article.
-
Palbociclib suppresses the cancer stem cell properties and cell proliferation through increased levels of miR-506 or miR-150 in Panc-1 and MiaPaCa-2 cells.Turk J Biol. 2022 Jul 18;46(5):342-360. doi: 10.55730/1300-0152.2622. eCollection 2022. Turk J Biol. 2022. PMID: 37529006 Free PMC article.
References
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. 10.1016/S0140-6736(14)60460-8 . - DOI - PMC - PubMed
-
- Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22(8):1266–72. . - PubMed
-
- Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(4):843–52. 10.1093/annonc/mdr398 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

