Development and Characterization of Acellular Extracellular Matrix Scaffolds from Porcine Menisci for Use in Cartilage Tissue Engineering
- PMID: 25919905
- PMCID: PMC4553380
- DOI: 10.1089/ten.TEC.2015.0036
Development and Characterization of Acellular Extracellular Matrix Scaffolds from Porcine Menisci for Use in Cartilage Tissue Engineering
Abstract
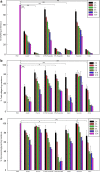
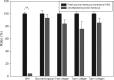
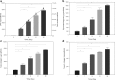
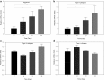
Given the growing number of arthritis patients and the limitations of current treatments, there is great urgency to explore cartilage substitutes by tissue engineering. In this study, we developed a novel decellularization method for menisci to prepare acellular extracellular matrix (ECM) scaffolds with minimal adverse effects on the ECM. Among all the acid treatments, formic acid treatment removed most of the cellular contents and preserved the highest ECM contents in the decellularized porcine menisci. Compared with fresh porcine menisci, the content of DNA decreased to 4.10%±0.03%, and there was no significant damage to glycosaminoglycan (GAG) or collagen. Histological staining also confirmed the presence of ECM and the absence of cellularity. In addition, a highly hydrophilic scaffold with three-dimensional interconnected porous structure was fabricated from decellularized menisci tissue. Human chondrocytes showed enhanced cell proliferation and synthesis of chondrocyte ECM including type II collagen and GAG when cultured in this acellular scaffold. Moreover, the scaffold effectively supported chondrogenesis of human bone marrow-derived mesenchymal stem cells. Finally, in vivo implantation was conducted in rats to assess the biocompatibility of the scaffolds. No significant inflammatory response was observed. The acellular ECM scaffold provided a native environment for cells with diverse physiological functions to promote cell proliferation and new tissue formation. This study reported a novel way to prepare decellularized meniscus tissue and demonstrated the potential as scaffolds to support cartilage repair.
Figures








Similar articles
-
A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells.Biomaterials. 2008 May;29(15):2378-87. doi: 10.1016/j.biomaterials.2008.01.037. Epub 2008 Mar 4. Biomaterials. 2008. PMID: 18313139
-
Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix.Tissue Eng Part A. 2009 Feb;15(2):231-41. doi: 10.1089/ten.tea.2008.0253. Tissue Eng Part A. 2009. PMID: 18950290 Free PMC article.
-
Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications.Tissue Eng Part A. 2012 Nov;18(21-22):2195-209. doi: 10.1089/ten.TEA.2011.0705. Epub 2012 Jul 20. Tissue Eng Part A. 2012. PMID: 22690787
-
The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage.Stem Cell Rev Rep. 2017 Feb;13(1):50-67. doi: 10.1007/s12015-016-9699-8. Stem Cell Rev Rep. 2017. PMID: 27826794 Review.
-
Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair.Int J Artif Organs. 2021 Apr;44(4):269-281. doi: 10.1177/0391398820953866. Epub 2020 Sep 18. Int J Artif Organs. 2021. PMID: 32945220 Review.
Cited by
-
Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo.Acta Biomater. 2018 Mar 15;69:71-82. doi: 10.1016/j.actbio.2017.12.043. Epub 2018 Jan 6. Acta Biomater. 2018. PMID: 29317369 Free PMC article.
-
Surface modification of decellularized trachea matrix with collagen and laser micropore technique to promote cartilage regeneration.Am J Transl Res. 2019 Sep 15;11(9):5390-5403. eCollection 2019. Am J Transl Res. 2019. PMID: 31632518 Free PMC article.
-
Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Anti-Tumor Immunity.Pharmaceutics. 2019 Oct 26;11(11):553. doi: 10.3390/pharmaceutics11110553. Pharmaceutics. 2019. PMID: 31717826 Free PMC article.
-
Allografts for partial meniscus repair: an in vitro and ex vivo meniscus culture study.Front Bioeng Biotechnol. 2023 Oct 12;11:1268176. doi: 10.3389/fbioe.2023.1268176. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37901839 Free PMC article.
-
Natural biopolymer scaffold for meniscus tissue engineering.Front Bioeng Biotechnol. 2022 Sep 30;10:1003484. doi: 10.3389/fbioe.2022.1003484. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246362 Free PMC article. Review.
References
-
- Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M., Kington R.S., Lane N.E., Nevitt M.C., Zhang Y., Sowers M., McAlindon T., Spector T.D., Poole A.R., Yanovski S.Z., Ateshian G., Sharma L., Buckwalter J.A., Brandt K.D., and Fries J.F. Osteoarthritis: new insights—part 1: the disease and its risk factors. Ann Intern Med 133, 635, 2000 - PubMed
-
- Felson D.T., and Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 41, 1343, 1998 - PubMed
-
- Hootman J.M., and Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 54, 226, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

