Effects of growth hormone therapeutic supplementation on hematopoietic stem/progenitor cells in children with growth hormone deficiency: focus on proliferation and differentiation capabilities
- PMID: 25920498
- PMCID: PMC4546702
- DOI: 10.1007/s12020-015-0591-0
Effects of growth hormone therapeutic supplementation on hematopoietic stem/progenitor cells in children with growth hormone deficiency: focus on proliferation and differentiation capabilities
Abstract
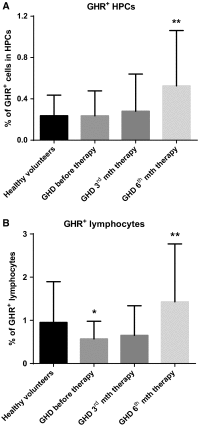
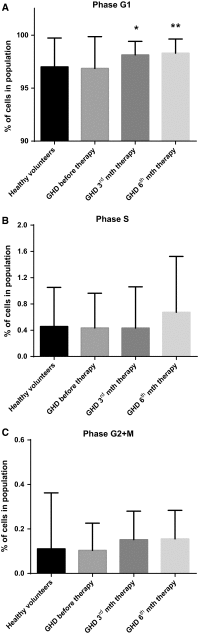
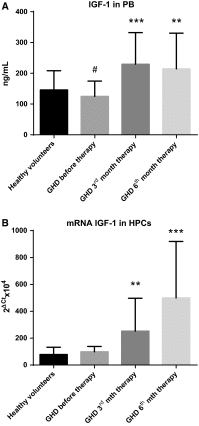
We investigated the direct effects of growth hormone (GH) replacement therapy (GH-RT) on hematopoiesis in children with GH deficiency (GHD) with the special emphasis on proliferation and cell cycle regulation. Peripheral blood (PB) was collected from sixty control individuals and forty GHD children before GH-RT and in 3rd and 6th month of GH-RT to measure hematological parameters and isolate CD34(+)-enriched hematopoietic progenitor cells (HPCs). Selected parameters of PB were analyzed by hematological analyzer. Moreover, collected HPCs were used to analyze GH receptor (GHR) and IGF1 expression, clonogenicity, and cell cycle activity. Finally, global gene expression profile of collected HPCs was analyzed using genome-wide RNA microarrays. GHD resulted in a decrease in several hematological parameters related to RBCs and significantly diminished clonogenicity of erythroid progenies. In contrast, GH-RT stimulated increases in clonogenic growth of erythroid lineage and RBC counts as well as significant up-regulation of cell cycle-propagating genes, including MAP2K1, cyclins D1/E1, PCNA, and IGF1. Likewise, GH-RT significantly modified GHR expression in isolated HPCs and augmented systemic IGF1 levels. Global gene expression analysis revealed significantly higher expression of genes associated with cell cycle, proliferation, and differentiation in HPCs from GH-treated subjects. (i) GH-RT significantly augments cell cycle progression in HPCs and increases clonogenicity of erythroid progenitors; (ii) GHR expression in HPCs is modulated by GH status; (iii) molecular mechanisms by which GH influences hematopoiesis might provide a basis for designing therapeutic interventions for hematological complications related to GHD.
Figures



Similar articles
-
The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency.Int J Mol Sci. 2017 Jan 7;18(1):111. doi: 10.3390/ijms18010111. Int J Mol Sci. 2017. PMID: 28067847 Free PMC article.
-
SNPs within the GH-signaling pathway are associated with the early IGF1 response to GH replacement therapy in GHD adults.Eur J Endocrinol. 2013 Nov 25;170(1):101-7. doi: 10.1530/EJE-13-0685. Print 2014 Jan. Eur J Endocrinol. 2013. PMID: 24114431 Clinical Trial.
-
The exon 3-deleted/full-length growth hormone receptor polymorphism and response to growth hormone therapy in growth hormone deficiency and Turner syndrome: a multicenter study.Horm Res Paediatr. 2012;77(2):85-93. doi: 10.1159/000335172. Epub 2012 Mar 23. Horm Res Paediatr. 2012. PMID: 22456308
-
Genetics of GHRH, GHRH-receptor, GH and GH-receptor: its impact on pharmacogenetics.Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):25-41. doi: 10.1016/j.beem.2010.06.006. Best Pract Res Clin Endocrinol Metab. 2011. PMID: 21396573 Review.
-
Association of growth hormone deficiency (GHD) with anxiety and depression: experimental data and evidence from GHD children and adolescents.Hormones (Athens). 2021 Dec;20(4):679-689. doi: 10.1007/s42000-021-00306-1. Epub 2021 Jul 1. Hormones (Athens). 2021. PMID: 34195937 Review.
Cited by
-
Apoptosis Evaluation in Circulating CD34+-Enriched Hematopoietic Stem and Progenitor Cells in Patients with Abnormally Increased Production of Endogenous Glucocorticoids in Course of Cushing's Syndrome.Int J Mol Sci. 2022 Dec 13;23(24):15794. doi: 10.3390/ijms232415794. Int J Mol Sci. 2022. PMID: 36555435 Free PMC article.
-
Iron Homeostasis and Hepcidin Concentration in Patients With Acromegaly.Front Endocrinol (Lausanne). 2022 Feb 8;12:788247. doi: 10.3389/fendo.2021.788247. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35211089 Free PMC article.
-
An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency.Exp Ther Med. 2016 May;11(5):1647-1652. doi: 10.3892/etm.2016.3091. Epub 2016 Feb 19. Exp Ther Med. 2016. PMID: 27168784 Free PMC article.
-
Long-term effects of growth hormone (GH) replacement therapy on hematopoiesis in a large cohort of children with GH deficiency.Endocrine. 2016 Jul;53(1):192-8. doi: 10.1007/s12020-015-0781-9. Epub 2015 Oct 28. Endocrine. 2016. PMID: 26511947
-
Influence of Growth Hormone and Glutamine on Intestinal Stem Cells: A Narrative Review.Nutrients. 2019 Aug 17;11(8):1941. doi: 10.3390/nu11081941. Nutrients. 2019. PMID: 31426533 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

