Myofiber damage precedes macrophage infiltration after in vivo injury in dysferlin-deficient A/J mouse skeletal muscle
- PMID: 25920768
- PMCID: PMC4450316
- DOI: 10.1016/j.ajpath.2015.02.020
Myofiber damage precedes macrophage infiltration after in vivo injury in dysferlin-deficient A/J mouse skeletal muscle
Erratum in
-
CORRECTION.Am J Pathol. 2024 Mar;194(3):471. doi: 10.1016/j.ajpath.2023.12.004. Epub 2024 Jan 2. Am J Pathol. 2024. PMID: 38365346 Free PMC article. No abstract available.
Abstract
Mutations in the dysferlin gene (DYSF) lead to human muscular dystrophies known as dysferlinopathies. The dysferlin-deficient A/J mouse develops a mild myopathy after 6 months of age, and when younger models the subclinical phase of the human disease. We subjected the tibialis anterior muscle of 3- to 4-month-old A/J mice to in vivo large-strain injury (LSI) from lengthening contractions and studied the progression of torque loss, myofiber damage, and inflammation afterward. We report that myofiber damage in A/J mice occurs before inflammatory cell infiltration. Peak edema and inflammation, monitored by magnetic resonance imaging and by immunofluorescence labeling of neutrophils and macrophages, respectively, develop 24 to 72 hours after LSI, well after the appearance of damaged myofibers. Cytokine profiles 72 hours after injury are consistent with extensive macrophage infiltration. Dysferlin-sufficient A/WySnJ mice show much less myofiber damage and inflammation and lesser cytokine levels after LSI than do A/J mice. Partial suppression of macrophage infiltration by systemic administration of clodronate-incorporated liposomes fails to suppress LSI-induced damage or to accelerate torque recovery in A/J mice. The findings from our studies suggest that, although macrophage infiltration is prominent in dysferlin-deficient A/J muscle after LSI, it is the consequence and not the cause of progressive myofiber damage.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





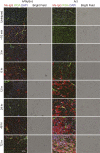
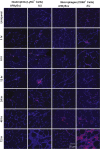


Similar articles
-
Distinct effects of contraction-induced injury in vivo on four different murine models of dysferlinopathy.J Biomed Biotechnol. 2012;2012:134031. doi: 10.1155/2012/134031. Epub 2012 Feb 6. J Biomed Biotechnol. 2012. PMID: 22431915 Free PMC article.
-
Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries.Am J Physiol Cell Physiol. 2010 Feb;298(2):C298-312. doi: 10.1152/ajpcell.00122.2009. Epub 2009 Nov 18. Am J Physiol Cell Physiol. 2010. PMID: 19923419 Free PMC article.
-
Genetic manipulation of dysferlin expression in skeletal muscle: novel insights into muscular dystrophy.Am J Pathol. 2009 Nov;175(5):1817-23. doi: 10.2353/ajpath.2009.090107. Epub 2009 Oct 15. Am J Pathol. 2009. PMID: 19834057 Free PMC article.
-
Dysferlin function in skeletal muscle: Possible pathological mechanisms and therapeutical targets in dysferlinopathies.Exp Neurol. 2016 Sep;283(Pt A):246-54. doi: 10.1016/j.expneurol.2016.06.026. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349407 Review.
-
Muscular dystrophy in dysferlin-deficient mouse models.Neuromuscul Disord. 2013 May;23(5):377-87. doi: 10.1016/j.nmd.2013.02.004. Epub 2013 Mar 7. Neuromuscul Disord. 2013. PMID: 23473732 Review.
Cited by
-
Identification of Serum Interleukin 6 Levels as a Disease Severity Biomarker in Facioscapulohumeral Muscular Dystrophy.J Neuromuscul Dis. 2022;9(1):83-93. doi: 10.3233/JND-210711. J Neuromuscul Dis. 2022. PMID: 34459413 Free PMC article.
-
Elevated Ca2+ at the triad junction underlies dysregulation of Ca2+ signaling in dysferlin-null skeletal muscle.Front Physiol. 2022 Nov 3;13:1032447. doi: 10.3389/fphys.2022.1032447. eCollection 2022. Front Physiol. 2022. PMID: 36406982 Free PMC article.
-
Upregulated IL-1β in dysferlin-deficient muscle attenuates regeneration by blunting the response to pro-inflammatory macrophages.Skelet Muscle. 2015 Aug 7;5:24. doi: 10.1186/s13395-015-0048-4. eCollection 2015. Skelet Muscle. 2015. PMID: 26251696 Free PMC article.
-
Phenotypic Drug Screening for Dysferlinopathy Using Patient-Derived Induced Pluripotent Stem Cells.Stem Cells Transl Med. 2019 Oct;8(10):1017-1029. doi: 10.1002/sctm.18-0280. Epub 2019 Jun 28. Stem Cells Transl Med. 2019. PMID: 31250983 Free PMC article.
-
Transcriptome analysis of skeletal muscle in dermatomyositis, polymyositis, and dysferlinopathy, using a bioinformatics approach.Front Neurol. 2023 Dec 6;14:1328547. doi: 10.3389/fneur.2023.1328547. eCollection 2023. Front Neurol. 2023. PMID: 38125829 Free PMC article.
References
-
- Lostal W., Bartoli M., Bourg N., Roudaut C., Bentaib A., Miyake K., Guerchet N., Fougerousse F., McNeil P., Richard I. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet. 2010;19:1897–1907. - PubMed
-
- Lostal W., Bartoli M., Roudaut C., Bourg N., Krahn M., Pryadkina M., Borel P., Suel L., Roche J.A., Stockholm D., Bloch R.J., Levy N., Bashir R., Richard I. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PLoS One. 2012;7:e38036. - PMC - PubMed
-
- Wein N., Avril A., Bartoli M., Beley C., Chaouch S., Laforet P., Behin A., Butler-Browne G., Mouly V., Krahn M., Garcia L., Levy N. Efficient bypass of mutations in dysferlin deficient patient cells by antisense-induced exon skipping. Hum Mutat. 2010;31:136–142. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

