Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy
- PMID: 25920797
- PMCID: PMC4523493
- DOI: 10.1016/j.semcancer.2015.04.002
Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy
Abstract
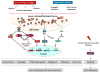
A variety of cell intrinsic or extrinsic stresses evoke perturbations in the folding environment of the endoplasmic reticulum (ER), collectively known as ER stress. Adaptation to stress and re-establishment of ER homeostasis is achieved by activation of an integrated signal transduction pathway called the unfolded protein response (UPR). Both ER stress and UPR activation have been implicated in a variety of human cancers. Although at early stages or physiological conditions of ER stress, the UPR generally promotes survival, when the stress becomes more stringent or prolonged, its role can switch to a pro-cell death one. Here, we discuss historical and recent evidence supporting an involvement of the UPR in malignancy, describe the main mechanisms by which tumor cells overcome ER stress to promote their survival, tumor progression and metastasis and discuss the current state of efforts to develop therapeutic approaches of targeting the UPR.
Keywords: Cancer; ER stress; Therapeutic approaches; Tumorigenesis; UPR.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Controlling the unfolded protein response-mediated life and death decisions in cancer.Semin Cancer Biol. 2015 Aug;33:57-66. doi: 10.1016/j.semcancer.2015.03.003. Epub 2015 Mar 23. Semin Cancer Biol. 2015. PMID: 25814342 Review.
-
Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer.Biochim Biophys Acta Rev Cancer. 2017 Jan;1867(1):58-66. doi: 10.1016/j.bbcan.2016.12.002. Epub 2016 Dec 15. Biochim Biophys Acta Rev Cancer. 2017. PMID: 27988298 Free PMC article. Review.
-
Targeting ER stress induced apoptosis and inflammation in cancer.Cancer Lett. 2013 May 28;332(2):249-64. doi: 10.1016/j.canlet.2010.07.016. Epub 2010 Aug 21. Cancer Lett. 2013. PMID: 20732741 Review.
-
ER proteostasis addiction in cancer biology: Novel concepts.Semin Cancer Biol. 2015 Aug;33:40-7. doi: 10.1016/j.semcancer.2015.04.003. Epub 2015 Apr 27. Semin Cancer Biol. 2015. PMID: 25931388 Review.
-
Endoplasmic reticulum proteostasis: a key checkpoint in cancer.Am J Physiol Cell Physiol. 2017 Feb 1;312(2):C93-C102. doi: 10.1152/ajpcell.00266.2016. Epub 2016 Nov 16. Am J Physiol Cell Physiol. 2017. PMID: 27856431 Free PMC article. Review.
Cited by
-
Suppression of ER-stress induction of GRP78 as an anti-neoplastic mechanism of the cardiac glycoside Lanatoside C in pancreatic cancer: Lanatoside C suppresses GRP78 stress induction.Neoplasia. 2021 Dec;23(12):1213-1226. doi: 10.1016/j.neo.2021.10.004. Epub 2021 Nov 9. Neoplasia. 2021. PMID: 34768108 Free PMC article.
-
Dual RNase activity of IRE1 as a target for anticancer therapies.J Cell Commun Signal. 2023 Dec;17(4):1145-1161. doi: 10.1007/s12079-023-00784-5. Epub 2023 Sep 18. J Cell Commun Signal. 2023. PMID: 37721642 Free PMC article. Review.
-
Tumor-Secreted GRP78 Promotes the Establishment of a Pre-metastatic Niche in the Liver Microenvironment.Front Immunol. 2020 Sep 29;11:584458. doi: 10.3389/fimmu.2020.584458. eCollection 2020. Front Immunol. 2020. PMID: 33133103 Free PMC article.
-
ONC201: Stressing tumors to death.Sci Signal. 2016 Feb 16;9(415):fs1. doi: 10.1126/scisignal.aad7955. Sci Signal. 2016. PMID: 26884598 Free PMC article.
-
Spirocyclic dimer SpiD7 activates the unfolded protein response to selectively inhibit growth and induce apoptosis of cancer cells.J Biol Chem. 2022 May;298(5):101890. doi: 10.1016/j.jbc.2022.101890. Epub 2022 Apr 1. J Biol Chem. 2022. PMID: 35378132 Free PMC article.
References
-
- Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–46. - PubMed
-
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. - PubMed
-
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–73. - PubMed
-
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. - PubMed
-
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

