Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis
- PMID: 25921340
- PMCID: PMC4626445
- DOI: 10.1038/mi.2015.34
Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis
Abstract
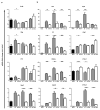
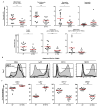
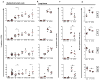
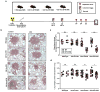
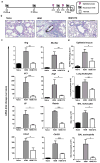
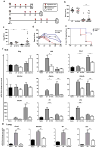
The roles of macrophages in type 2-driven inflammation and fibrosis remain unclear. Here, using CD11b-diphtheria toxin receptor (DTR) transgenic mice and three models of interleukin 13 (IL-13)-dependent inflammation, fibrosis, and immunity, we show that CD11b(+) F4/80(+) Ly6C(+) macrophages are required for the maintenance of type 2 immunity within affected tissues but not secondary lymphoid organs. Direct depletion of macrophages during the maintenance or resolution phases of secondary Schistosoma mansoni egg-induced granuloma formation caused a profound decrease in inflammation, fibrosis, and type 2 gene expression. Additional studies with CD11c-DTR and CD11b/CD11c-DTR double-transgenic mice suggested that macrophages but not dendritic cells were critical. Mechanistically, macrophage depletion impaired effector CD4(+) T helper type 2 (Th2) cell homing and activation within the inflamed lung. Depletion of CD11b(+) F4/80(+) Ly6C(+) macrophages similarly reduced house dust mite-induced allergic lung inflammation and suppressed IL-13-dependent immunity to the nematode parasite Nippostrongylus brasiliensis. Consequently, therapeutic strategies targeting macrophages offer a novel approach to ameliorate established type 2 inflammatory diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discovery medicine. 2011;11:459–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

