A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims
- PMID: 25922328
- PMCID: PMC7103985
- DOI: 10.1016/j.jegh.2014.08.002
A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims
Abstract
Background: Cost-effective interventions are needed to control the transmission of viral respiratory tract infections (RTIs) in mass gatherings. Facemasks are a promising preventive measure, however, previous studies on the efficacy of facemasks have been inconclusive. This study proposes a large-scale facemask trial during the Hajj pilgrimage in Saudi Arabia and presents this protocol to illustrate its feasibility and to promote both collaboration with other research groups and additional relevant studies.
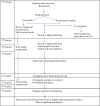
Methods/design: A cluster-randomised controlled trial is being conducted to test the efficacy of standard facemasks in preventing symptomatic and proven viral RTIs among pilgrims during the Hajj season in Mina, Mecca, Saudi Arabia. The trial will compare the 'supervised use of facemasks' versus 'standard measures' among pilgrims over several Hajj seasons. Cluster-randomisation will be done by accommodation tents with a 1:1 ratio. For the intervention tents, free facemasks will be provided to be worn consistently for 7days. Data on flu-like symptoms and mask use will be recorded in diaries. Nasal samples will be collected from symptomatic recruits and tested for nucleic acid of respiratory viruses. Data obtained from questionnaires, diaries and laboratory tests will be analysed to examine whether mask use significantly reduces the frequency of laboratory-confirmed respiratory viral infection and syndromic RTI as primary outcomes.
Conclusions: This trial will provide valuable evidence on the efficacy of standard facemask use in preventing viral respiratory tract infections at mass gatherings. This study is registered at the Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN: ACTRN12613001018707 (http://www.anzctr.org.au).
Keywords: Facemask; Hajj pilgrimage; Influenza; Middle East respiratory syndrome coronavirus; Viral respiratory tract infection.
Copyright © 2014 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Professor Robert Booy and Dr. Leon Heron have received funding from Baxter, CSL, GSK, Merck, Novartis, Pfizer, Roche, Romark and Sanofi Pasteur for the conduct of sponsored research, travel to present at conferences or consultancy work; all funding received are directed to research accounts at The Children’s Hospital at Westmead. Dr. Iman Ridda has received Grants for investigator-driven research from GSK and for consultation from Merck. The other authors have declared no conflict of interest in relation to this work.
Figures

References
-
- World Health Organization Global influenza programme: pandemic influenza preparedness and response, WHO guidance document 2009. Available from: http://www.who.int/influenza/resources/documents/pandemic_guidance_04_20... (accessed 4 July, 2014). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
