Regulation of correlative inhibition of axillary bud outgrowth by basal branches varies with growth stage in Trifolium repens
- PMID: 25922495
- PMCID: PMC4473983
- DOI: 10.1093/jxb/erv184
Regulation of correlative inhibition of axillary bud outgrowth by basal branches varies with growth stage in Trifolium repens
Abstract
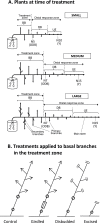
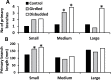
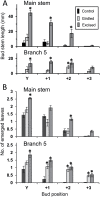
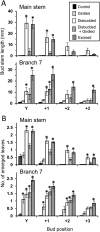
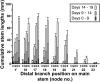
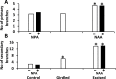
In Trifolium repens the decline in bud outgrowth that occurs with distance from basal root systems is due to correlative inhibition by the first-formed basal branches. The apical and axillary buds on these basal branches are the source of the inhibitory effect, but their mode of action is unclear. Inhibition might occur via basal branches being a sink for xylem-transported branching stimulants or alternatively by providing a source of inhibitory signals, or by both mechanisms. To distinguish between these mechanisms, four experiments were conducted on plants varying in initial growth stage from 10 to 19 nodes along their main stems to determine any variation in the relative importance of the operative mechanisms of correlative inhibition. Inhibitory signal exported into the main stem, detected as a branching response to girdling of basal branches, was relatively more significant in smaller (initially with 10-15 nodes on the main stem) than in larger (>19 nodes on main stem) plants. This signal was shown not to involve auxin fluxes, and is unidentified. However, across all stages of growth, the predominant mechanism driving correlative inhibition was the action of axillary and apical buds of basal branches as sinks for the stimulatory signal. This study indicates that the relative importance of the mechanisms regulating bud outgrowth in T. repens varies with growth stage and that, during intermediate stages, regulation has some similarity to that in Pisum.
Keywords: Apical dominance; auxin; branching regulation; correlative inhibition; cytokinin; prostrate clonal herbs; white clover (Trifolium repens)..
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures

References
-
- Brewer PB, Koltai H, Beveridge CA. 2013. Diverse roles of strigolactones in plant development. Molecular Plant 6, 18–28. - PubMed
-
- Carlson GE. 1966. Growth of white clover leaves – developmental morphology and parameters at ten stages. Crop Science 6, 293–294.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

