NOD-Like Receptor Signaling in Cholesteatoma
- PMID: 25922834
- PMCID: PMC4398947
- DOI: 10.1155/2015/408169
NOD-Like Receptor Signaling in Cholesteatoma
Abstract
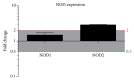
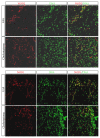
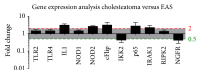
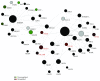
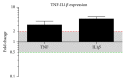
Background. Cholesteatoma is a destructive process of the middle ear resulting in erosion of the surrounding bony structures with consequent hearing loss, vestibular dysfunction, facial paralysis, or intracranial complications. The etiopathogenesis of cholesteatoma is controversial but is associated with recurrent ear infections. The role of intracellular innate immune receptors, the NOD-like receptors, and their associated signaling networks was investigated in cholesteatoma, since mutations in NOD-like receptor-related genes have been implicated in other chronic inflammatory disorders. Results. The expression of NOD2 mRNA and protein was significantly induced in cholesteatoma compared to the external auditory canal skin, mainly located in the epithelial layer of cholesteatoma. Microarray analysis showed significant upregulation for NOD2, not for NOD1, TLR2, or TLR4 in cholesteatoma. Moreover, regulation of genes in an interaction network of the NOD-adaptor molecule RIPK2 was detected. In addition to NOD2, NLRC4, and PYCARD, the downstream molecules IRAK1 and antiapoptotic regulator CFLAR showed significant upregulation, whereas SMAD3, a proapoptotic inducer, was significantly downregulated. Finally, altered regulation of inflammatory target genes of NOD signaling was detected. Conclusions. These results indicate that the interaction of innate immune signaling mediated by NLRs and their downstream target molecules is involved in the etiopathogenesis and growth of cholesteatoma.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

