SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors
- PMID: 25923013
- PMCID: PMC4413894
- DOI: 10.1038/srep09841
SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors
Abstract
Metastasis is responsible for over 90% of cancer-associated mortality. In epithelial carcinomas, a key process in metastatic progression is the epigenetic reprogramming of an epithelial-to-mesenchymal transition-like (EMT) change towards invasive cellular phenotypes. In non-epithelial cancers, different mechanisms must underlie metastatic change, but relatively little is known about the factors involved. Here, we identify the chromatin regulatory Sirtuin factor SIRT7 as a key regulator of metastatic phenotypes in both epithelial and mesenchymal cancer cells. In epithelial prostate carcinomas, high SIRT7 levels are associated with aggressive cancer phenotypes, metastatic disease, and poor patient prognosis, and depletion of SIRT7 can reprogram these cells to a less aggressive phenotype. Interestingly, SIRT7 is also important for maintaining the invasiveness and metastatic potential of non-epithelial sarcoma cells. Moreover, SIRT7 inactivation dramatically suppresses cancer cell metastasis in vivo, independent of changes in primary tumor growth. Mechanistically, we also uncover a novel link between SIRT7 and its family member SIRT1, providing the first demonstration of direct interaction and functional interplay between two mammalian sirtuins. Together with previous work, our findings highlight the broad role of SIRT7 in maintaining the metastatic cellular phenotype in diverse cancers.
Conflict of interest statement
Dr. Chua’s research is partly funded by Daiichi Sankyo company. Dr. Michishita-Kioi, Dr. Tanaka, and Dr. Aonuma are employees of Daiichi Sankyo Group Companies.
Figures

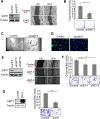

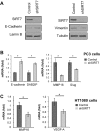

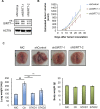
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

