Effects of mechanical stretching on the morphology and cytoskeleton of vaginal fibroblasts from women with pelvic organ prolapse
- PMID: 25923074
- PMCID: PMC4463595
- DOI: 10.3390/ijms16059406
Effects of mechanical stretching on the morphology and cytoskeleton of vaginal fibroblasts from women with pelvic organ prolapse
Abstract
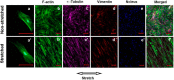
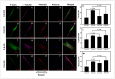
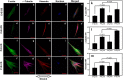
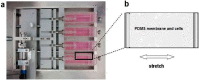
Mechanical load and postmenopausal hypoestrogen are risk factors for pelvic organ prolapse (POP). In this study, we applied a 0.1-Hz uniaxial cyclic mechanical stretching (CS) with 10% elongation and 10⁻⁸ M 17-β-estradiol to vaginal fibroblasts isolated from postmenopausal women with or without POP to investigate the effects of CS and estrogen on cell morphology and cytoskeletons of normal and POP fibroblasts. Under static culture condition, POP fibroblasts exhibited lower cell circularity and higher relative fluorescence intensities (RFIs) of F-actin, α-tubulin and vimentin. When cultured with CS, all fibroblasts grew perpendicular to the force and exhibited a decreased cell projection area, cell circularity and increased cell length/width ratio; normal fibroblasts exhibited increased RFIs of all three types of cytoskeleton, and POP fibroblasts exhibited a decreased RFI of F-actin and no significant differences of α-tubulin and vimentin. After being cultured with 17-β-estradiol and CS, normal fibroblasts no longer exhibited significant changes in the cell projection area and the RFIs of F-actin and α-tubulin; POP fibroblasts exhibited no significant changes in cell circularity, length/width ratio and F-actin even with the increased RFIs of α-tubulin and vimentin. These findings suggest that POP fibroblasts have greater sensitivity to and lower tolerance for mechanical stretching, and estrogen can improve the prognosis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

