A comparison of some organizational characteristics of the mouse central retina and the human macula
- PMID: 25923208
- PMCID: PMC4414478
- DOI: 10.1371/journal.pone.0125631
A comparison of some organizational characteristics of the mouse central retina and the human macula
Abstract
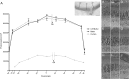
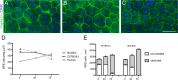
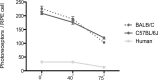
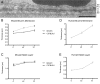
Mouse models have greatly assisted our understanding of retinal degenerations. However, the mouse retina does not have a macula, leading to the question of whether the mouse is a relevant model for macular degeneration. In the present study, a quantitative comparison between the organization of the central mouse retina and the human macula was made, focusing on some structural characteristics that have been suggested to be important in predisposing the macula to stresses leading to degeneration: photoreceptor density, phagocytic load on the RPE, and the relative thinness of Bruch's membrane. Light and electron microscopy measurements from retinas of two strains of mice, together with published data on human retinas, were used for calculations and subsequent comparisons. As in the human retina, the central region of the mouse retina possesses a higher photoreceptor cell density and a thinner Bruch's membrane than in the periphery; however, the magnitudes of these periphery to center gradients are larger in the human. Of potentially greater relevance is the actual photoreceptor cell density, which is much greater in the mouse central retina than in the human macula, underlying a higher phagocytic load for the mouse RPE. Moreover, at eccentricities that correspond to the peripheral half of the human macula, the rod to cone ratio is similar between mouse and human. Hence, with respect to photoreceptor density and phagocytic load of the RPE, the central mouse retina models at least the more peripheral part of the macula, where macular degeneration is often first evident.
Conflict of interest statement
Figures
References
-
- Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002;109(10):1767–79. Epub 2002/10/03. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

