A Human Monoclonal Antibody against Hepatitis B Surface Antigen with Potent Neutralizing Activity
- PMID: 25923526
- PMCID: PMC4414269
- DOI: 10.1371/journal.pone.0125704
A Human Monoclonal Antibody against Hepatitis B Surface Antigen with Potent Neutralizing Activity
Abstract
We describe the production and characterization of human monoclonal antibodies (mAb) specific for the major hepatitis B virus (HBV) S protein. The mAbs, two IgG1κ and one IgG1λ, were secreted by B-cell clones obtained from peripheral blood mononuclear cells (PBMC) of one person convalescent from acute hepatitis B and one vaccinated individual. The former recognized a denaturation-insensitive epitope within the p24 protein whereas the latter recognized a denaturation-sensitive, conformational epitope located within the HBsAg common "a" determinant. This mAb, denominated ADRI-2F3, displayed a very high protective titer of over 43,000 IU/mg mAb and showed an extremely potent neutralizing activity in the in vitro model of HBV infection using primary hepatocytes from Tupaia belangeri as target. Recombinant variable heavy and light domain sequences derived from mAb ADRI-2F3 were cloned into eukaryotic expression vectors and showed identical fine specificity and 1 log10 higher titer than the original IgG1λ. It is envisaged that such mAb will be able to efficiently prevent HBV reinfection after liver transplantation for end-stage chronic HBV infection or infection after needle-stick exposure, providing an unlimited source of valuable protective anti-HBs antibody.
Conflict of interest statement
Figures
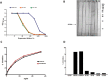

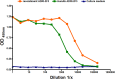
References
-
- Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol 2005;34: S1–S3. - PubMed
-
- World Health Organization (WHO). Hepatitis B: WHO fact sheet on Hepatitis B providing key facts and information. Fact sheet N°204. Updated March 2015 Available: http://www.who.int/mediacentre/factsheets/fs204/en/.
-
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336: 1855–1859. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

