Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth
- PMID: 25923988
- PMCID: PMC4418220
- DOI: 10.1038/ncomms8064
Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth
Abstract
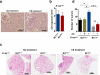
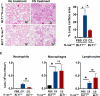
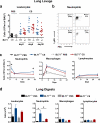
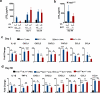
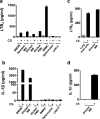
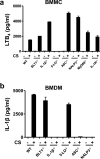
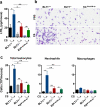
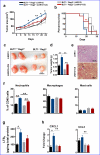
Chronic exposure to crystalline silica (CS) causes silicosis, an irreversible lung inflammatory disease that may eventually lead to lung cancer. In this study, we demonstrate that in K-ras(LA1) mice, CS exposure markedly enhances the lung tumour burden and genetic deletion of leukotriene B4 receptor-1 (BLT1(-/-)) attenuates this increase. Pulmonary neutrophilic inflammation induced by CS is significantly reduced in BLT1(-/-)K-ras(LA1) mice. CS exposure induces LTB4 production by mast cells and macrophages independent of inflammasome activation. In an air-pouch model, CS-induced neutrophil recruitment is dependent on LTB4 production by mast cells and BLT1 expression on neutrophils. In an implantable lung tumour model, CS exposure results in rapid tumour growth and decreased survival that is attenuated in the absence of BLT1. These results suggest that the LTB4/BLT1 axis sets the pace of CS-induced sterile inflammation that promotes lung cancer progression. This knowledge may facilitate development of immunotherapeutic strategies to fight silicosis and lung cancer.
Figures
References
-
- Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. - PubMed
-
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13(4):233–45. - PubMed
-
- Colotta F, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. - PubMed
-
- Russo M, Di Nicolantonio F, Bardelli A. Climbing RAS, the everest of oncogenes. Cancer Discov. 2014;4(1):19–21. - PubMed
-
- Ji H, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

