Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects
- PMID: 25924675
- PMCID: PMC4440597
- DOI: 10.1186/s12974-015-0299-3
Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects
Abstract
Background: The inflammatory mediator lipopolysaccharide (LPS) has been shown to induce acute gliosis in neonatal mice. However, the progressive effects on the murine neurodevelopmental program over the week that follows systemic inflammation are not known. Thus, we investigated the effects of repeated LPS administration in the first postnatal week in mice, a condition mimicking sepsis in late preterm infants, on the developing central nervous system (CNS).
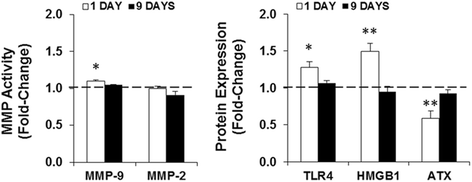
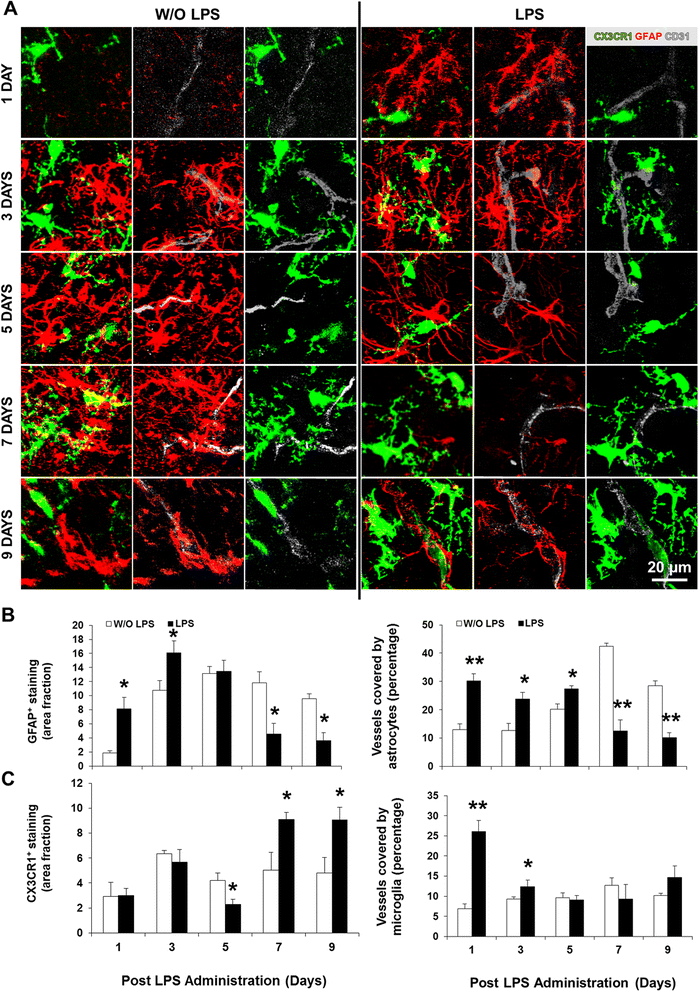
Methods: Systemic inflammation was induced by daily intraperitoneal administration (i.p.) of LPS (6 mg/kg) in newborn mice from postnatal day (PND) 4 to PND6. The effects on neurodevelopment were examined by staining the white matter and neurons with Luxol Fast Blue and Cresyl Violet, respectively. The inflammatory response was assessed by quantifying the expression/activity of matrix metalloproteinases (MMP), toll-like receptor (TLR)-4, high mobility group box (HMGB)-1, and autotaxin (ATX). In addition, B6 CX3CR1(gfp/+) mice combined with cryo-immunofluorescence were used to determine the acute, delayed, and lasting effects on myelination, microglia, and astrocytes.
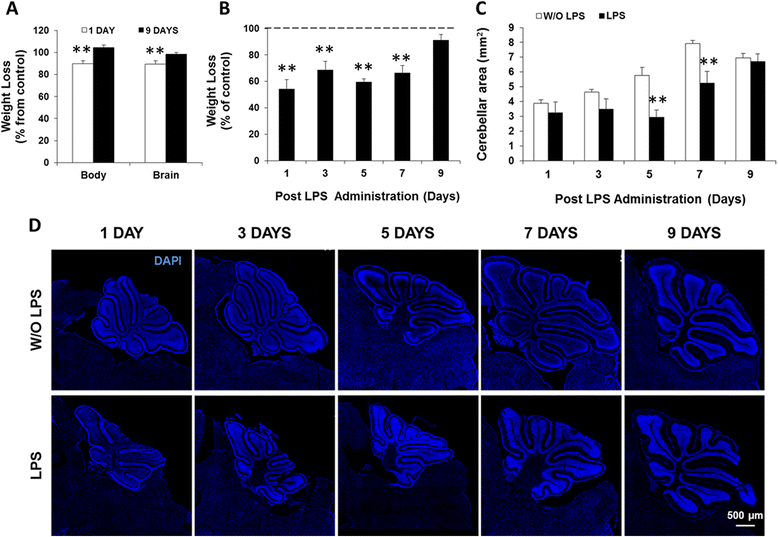
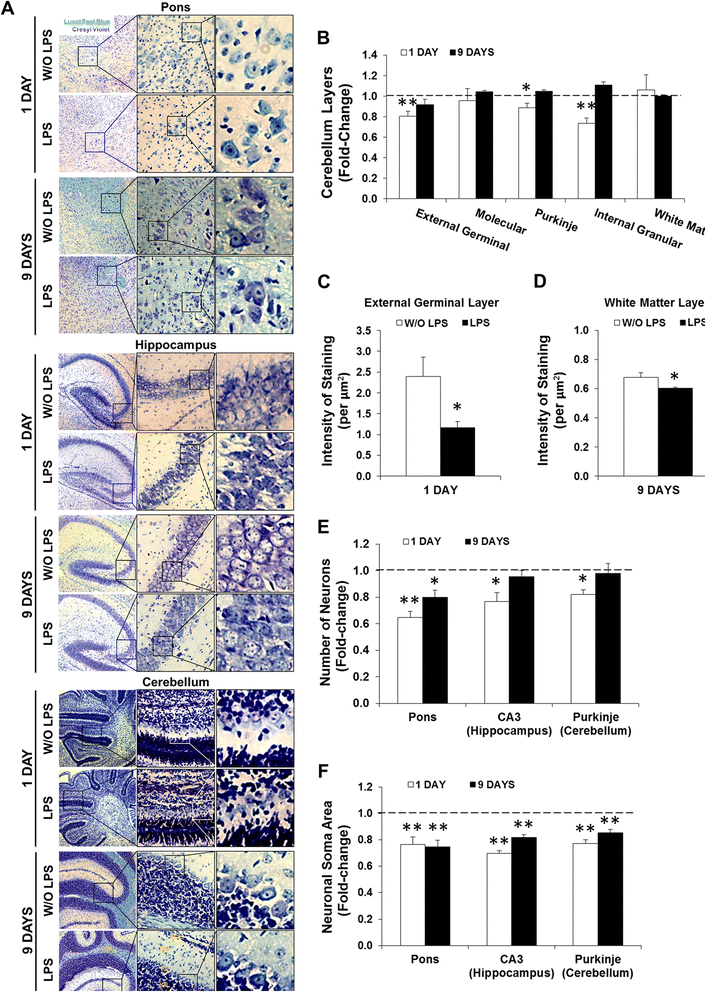
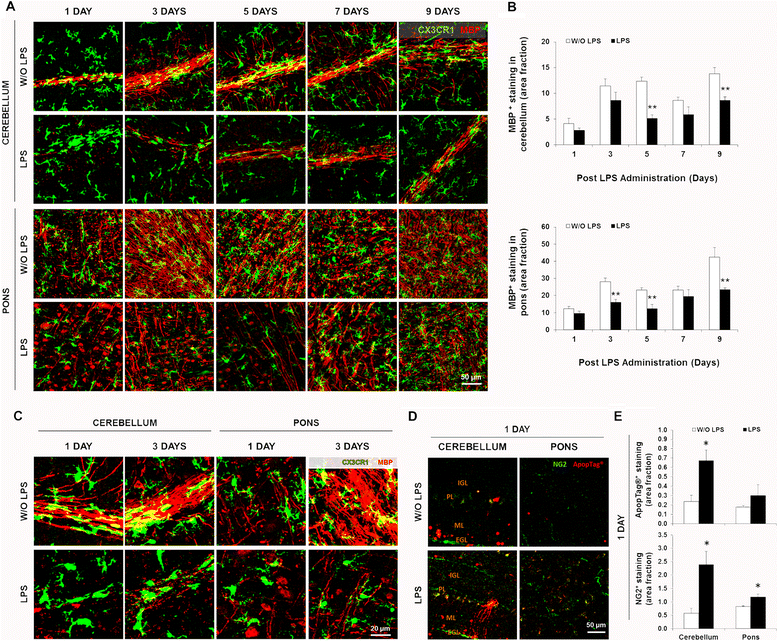
Results: LPS administration led to acute body and brain weight loss as well as overt structural changes in the brain such as cerebellar hypoplasia, neuronal loss/shrinkage, and delayed myelination. The impaired myelination was associated with alterations in the proliferation and differentiation of NG2 progenitor cells early after LPS administration, rather than with excessive phagocytosis by CNS myeloid cells. In addition to disruptions in brain architecture, a robust inflammatory response to LPS was observed. Quantification of inflammatory biomarkers revealed decreased expression of ATX with concurrent increases in HMGB1, TLR-4, and MMP-9 expression levels. Acute astrogliosis (GFAP(+) cells) in the brain parenchyma and at the microvasculature interface together with parenchymal microgliosis (CX3CR1(+) cells) were also observed. These changes preceded the migration/proliferation of CX3CR1(+) cells around the vessels at later time points and the subsequent loss of GFAP(+) astrocytes.
Conclusion: Collectively, our study has uncovered a complex innate inflammatory reaction and associated structural changes in the brains of neonatal mice challenged peripherally with LPS. These findings may explain some of the neurobehavioral abnormalities that develop following neonatal sepsis.
Figures



References
-
- Ogunlesi TA, Ogunfowora OB. Predictors of mortality in neonatal septicemia in an underresourced setting. J Natl Med Assoc. 2010;102(10):915–21. - PubMed
-
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

