Systematic review of model-based cervical screening evaluations
- PMID: 25924871
- PMCID: PMC4419493
- DOI: 10.1186/s12885-015-1332-8
Systematic review of model-based cervical screening evaluations
Abstract
Background: Optimising population-based cervical screening policies is becoming more complex due to the expanding range of screening technologies available and the interplay with vaccine-induced changes in epidemiology. Mathematical models are increasingly being applied to assess the impact of cervical cancer screening strategies.
Methods: We systematically reviewed MEDLINE®, Embase, Web of Science®, EconLit, Health Economic Evaluation Database, and The Cochrane Library databases in order to identify the mathematical models of human papillomavirus (HPV) infection and cervical cancer progression used to assess the effectiveness and/or cost-effectiveness of cervical cancer screening strategies. Key model features and conclusions relevant to decision-making were extracted.
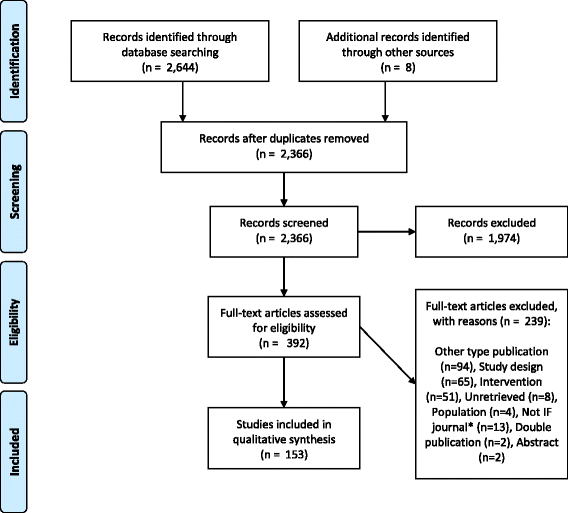
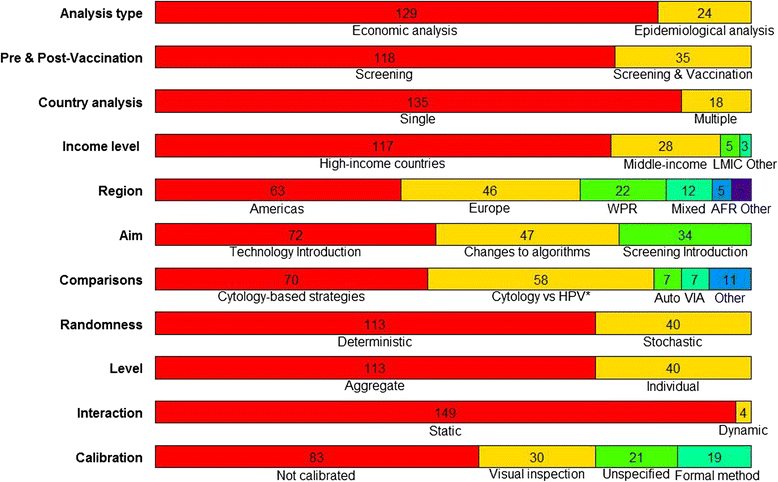
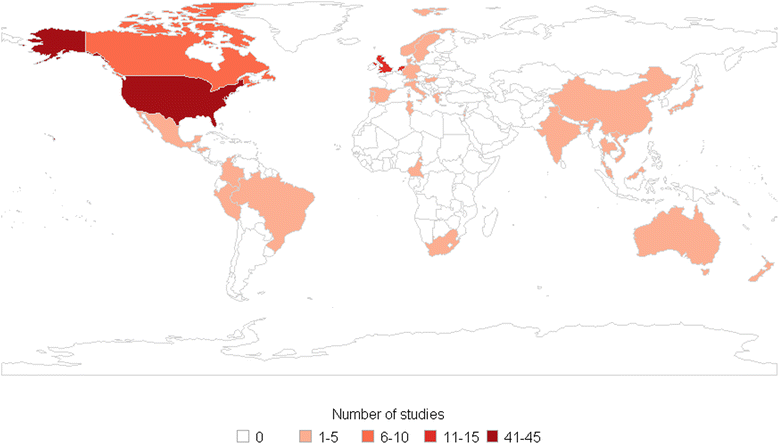
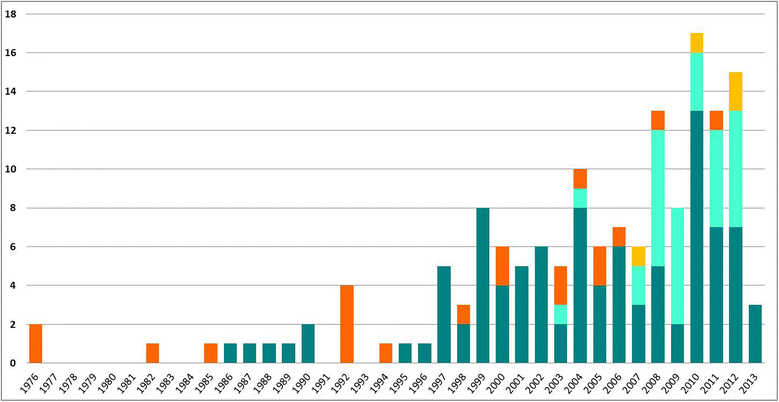
Results: We found 153 articles meeting our eligibility criteria published up to May 2013. Most studies (72/153) evaluated the introduction of a new screening technology, with particular focus on the comparison of HPV DNA testing and cytology (n = 58). Twenty-eight in forty of these analyses supported HPV DNA primary screening implementation. A few studies analysed more recent technologies - rapid HPV DNA testing (n = 3), HPV DNA self-sampling (n = 4), and genotyping (n = 1) - and were also supportive of their introduction. However, no study was found on emerging molecular markers and their potential utility in future screening programmes. Most evaluations (113/153) were based on models simulating aggregate groups of women at risk of cervical cancer over time without accounting for HPV infection transmission. Calibration to country-specific outcome data is becoming more common, but has not yet become standard practice.
Conclusions: Models of cervical screening are increasingly used, and allow extrapolation of trial data to project the population-level health and economic impact of different screening policy. However, post-vaccination analyses have rarely incorporated transmission dynamics. Model calibration to country-specific data is increasingly common in recent studies.
Figures
Similar articles
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
[Health technology assessment report. Use of liquid-based cytology for cervical cancer precursors screening].Epidemiol Prev. 2012 Sep-Oct;36(5 Suppl 2):e1-e33. Epidemiol Prev. 2012. PMID: 23139163 Italian.
-
The Utility of an Human Papillomavirus Genotype Assay for Cancer Screening in Self-Collected Urine and Vaginal Samples from Japanese Women.Gynecol Obstet Invest. 2025;90(2):143-152. doi: 10.1159/000541641. Epub 2024 Oct 7. Gynecol Obstet Invest. 2025. PMID: 39374596 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Positron emission tomography/computerised tomography imaging in detecting and managing recurrent cervical cancer: systematic review of evidence, elicitation of subjective probabilities and economic modelling.Health Technol Assess. 2013 Mar;17(12):1-323. doi: 10.3310/hta17120. Health Technol Assess. 2013. PMID: 23537558 Free PMC article.
Cited by
-
Cost-effective management of women with minor cervical lesions: Revisiting the application of HPV DNA testing.Gynecol Oncol. 2016 Nov;143(2):326-333. doi: 10.1016/j.ygyno.2016.08.231. Epub 2016 Aug 17. Gynecol Oncol. 2016. PMID: 27542966 Free PMC article.
-
Quantifying societal burden of radiation-induced small bowel toxicity in patients with rectal cancer.Front Oncol. 2024 Jul 8;14:1340081. doi: 10.3389/fonc.2024.1340081. eCollection 2024. Front Oncol. 2024. PMID: 39040451 Free PMC article.
-
HPV-FRAME: A consensus statement and quality framework for modelled evaluations of HPV-related cancer control.Papillomavirus Res. 2019 Dec;8:100184. doi: 10.1016/j.pvr.2019.100184. Epub 2019 Sep 7. Papillomavirus Res. 2019. PMID: 31505258 Free PMC article.
-
Implementation of cervical cancer prevention and screening across five tertiary hospitals in Nepal and its policy implications: A mixed-methods study.PLOS Glob Public Health. 2024 Jan 18;4(1):e0002832. doi: 10.1371/journal.pgph.0002832. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 38236836 Free PMC article.
-
Detecting Human Papillomavirus Type 16 in Cervical Cancer Patients with Molecular Variation of Gene L1 in Riau Province Indonesia.Asian Pac J Cancer Prev. 2022 Jan 1;23(1):87-92. doi: 10.31557/APJCP.2022.23.1.87. Asian Pac J Cancer Prev. 2022. PMID: 35092375 Free PMC article.
References
-
- Goldie SJ, Kim JJ, Myers E. Chapter 19: Cost-effectiveness of cervical cancer screening. Vaccine. 2006;24 Suppl 3:S3–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

