Estrogens Suppress Spinal Endomorphin 2 Release in Female Rats in Phase with the Estrous Cycle
- PMID: 25925013
- PMCID: PMC4575620
- DOI: 10.1159/000430817
Estrogens Suppress Spinal Endomorphin 2 Release in Female Rats in Phase with the Estrous Cycle
Abstract
Background/aims: Male and female rats differ in their ability to utilize spinal endomorphin 2 (EM2; the predominant mu-opioid receptor ligand in spinal cord) and in the mechanisms that underlie spinal EM2 analgesic responsiveness. We investigated the relevance of spinal estrogen receptors (ERs) to the in vivo regulation of spinal EM2 release.
Methods: ER antagonists were administered directly to the lumbosacral spinal cord of male and female rats, intrathecal perfusate was collected, and resulting changes in EM2 release were quantified using a plate-based radioimmunoassay.
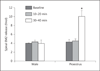
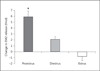
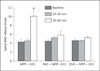
Results: Intrathecal application of an antagonist of either estrogen receptor-α (ERα) or the ER GPR30 failed to alter spinal EM2 release. Strikingly, however, the concomitant blockade of ERα and GPR30 enhanced spinal EM2 release. This effect was sexually dimorphic, being absent in males. Furthermore, the magnitude of the enhancement of spinal EM2 release in females was dependent upon estrous cycle stage, suggesting a relationship with circulating levels of 17β-estradiol. The rapid onset of enhanced EM2 release following intrathecal application of ERα/GPR30 antagonists (within 30-40 min) suggests mediation via ERs in the plasma membrane, not the nucleus. Notably, both ovarian and spinally synthesized estrogens are essential for membrane ER regulation of spinal EM2 release.
Conclusion: These findings underscore the importance of estrogens for the regulation of spinal EM2 activity and, by extension, endogenous spinal EM2 antinociception in females. Components of the spinal estrogenic mechanism(s) that suppress EM2 release could represent novel drug targets for improving utilization of endogenous spinal EM2, and thereby pain management in women.
© 2015 S. Karger AG, Basel.
Figures

Similar articles
-
Plasticity of Signaling by Spinal Estrogen Receptor α, κ-Opioid Receptor, and Metabotropic Glutamate Receptors over the Rat Reproductive Cycle Regulates Spinal Endomorphin 2 Antinociception: Relevance of Endogenous-Biased Agonism.J Neurosci. 2017 Nov 15;37(46):11181-11191. doi: 10.1523/JNEUROSCI.1927-17.2017. Epub 2017 Oct 12. J Neurosci. 2017. PMID: 29025923 Free PMC article.
-
Contribution of Endogenous Spinal Endomorphin 2 to Intrathecal Opioid Antinociception in Rats Is Agonist Dependent and Sexually Dimorphic.J Pain. 2015 Nov;16(11):1200-10. doi: 10.1016/j.jpain.2015.08.003. Epub 2015 Sep 2. J Pain. 2015. PMID: 26342648 Free PMC article.
-
Estrogens synthesized and acting within a spinal oligomer suppress spinal endomorphin 2 antinociception: ebb and flow over the rat reproductive cycle.Pain. 2017 Oct;158(10):1903-1914. doi: 10.1097/j.pain.0000000000000991. Pain. 2017. PMID: 28902684 Free PMC article.
-
Arbiters of endogenous opioid analgesia: role of CNS estrogenic and glutamatergic systems.Transl Res. 2021 Aug;234:31-42. doi: 10.1016/j.trsl.2021.02.002. Epub 2021 Feb 7. Transl Res. 2021. PMID: 33567346 Free PMC article. Review.
-
Exploiting endogenous opioids: Lessons learned from endomorphin 2 in the female rat.Peptides. 2019 Feb;112:133-138. doi: 10.1016/j.peptides.2018.12.002. Epub 2018 Dec 14. Peptides. 2019. PMID: 30557590 Free PMC article. Review.
Cited by
-
Sex differences in innate immunity and its impact on opioid pharmacology.J Neurosci Res. 2017 Jan 2;95(1-2):487-499. doi: 10.1002/jnr.23852. J Neurosci Res. 2017. PMID: 27870418 Free PMC article. Review.
-
Estrogens as arbiters of sex-specific and reproductive cycle-dependent opioid analgesic mechanisms.Vitam Horm. 2019;111:227-246. doi: 10.1016/bs.vh.2019.06.002. Epub 2019 Jul 2. Vitam Horm. 2019. PMID: 31421702 Free PMC article.
-
Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the μ-Opioid Receptor.J Pharmacol Exp Ther. 2016 Jun;357(3):509-19. doi: 10.1124/jpet.116.232421. Epub 2016 Apr 7. J Pharmacol Exp Ther. 2016. PMID: 27056847 Free PMC article.
-
Pain mechanisms in the transgender individual: a review.Front Pain Res (Lausanne). 2024 Mar 27;5:1241015. doi: 10.3389/fpain.2024.1241015. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38601924 Free PMC article. Review.
-
Plasticity of Signaling by Spinal Estrogen Receptor α, κ-Opioid Receptor, and Metabotropic Glutamate Receptors over the Rat Reproductive Cycle Regulates Spinal Endomorphin 2 Antinociception: Relevance of Endogenous-Biased Agonism.J Neurosci. 2017 Nov 15;37(46):11181-11191. doi: 10.1523/JNEUROSCI.1927-17.2017. Epub 2017 Oct 12. J Neurosci. 2017. PMID: 29025923 Free PMC article.
References
-
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. - PubMed
-
- Teepker M, Peters M, Vedder H, Schepelmann K, Lautenbacher S. Menstrual variation in experimental pain: correlation with gonadal hormones. Neuropsychobiology. 2010;61:131–140. - PubMed
-
- Ibironke GF, Aji KE. Pain threshold variations in female rats as a function of the estrus cycle. Niger J Physiol Sci. 2011;26:67–70. - PubMed
-
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

