Natural killer cell therapy in children with relapsed leukemia
- PMID: 25925135
- PMCID: PMC4634362
- DOI: 10.1002/pbc.25555
Natural killer cell therapy in children with relapsed leukemia
Abstract
Background: Novel therapies are needed for children with relapsed or refractory leukemia. We therefore tested the safety and feasibility of haploidentical natural killer cell therapy in this patient population.
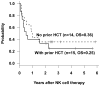
Procedure: Twenty-nine children who had relapsed or refractory leukemia were treated with chemotherapy followed by the infusion of haploidentical NK cells. Cohort 1 included 14 children who had not undergone prior allogeneic hematopoietic cell transplantation (HCT), whereas Cohort 2 included 15 children with leukemia that had relapsed after HCT.
Results: Twenty-six (90%) NK donors were KIR mismatched (14 with one KIR and 12 with 2 KIRs). The peak NK chimerism levels were >10% donor in 87% of the evaluable recipients. In Cohort 1, 10 had responsive disease and 12 proceeded to HCT thereafter. Currently, 5 (36%) are alive without leukemia. In Cohort 2, 10 had responsive disease after NK therapy and successfully proceeded to second HCT. At present, 4 (27%) are alive and leukemia-free. The NK cell infusions and the IL-2 injections were well-tolerated.
Conclusions: NK cell therapy is safe, feasible, and should be further investigated in patients with chemotherapy-resistant leukemia.
Keywords: NK cell; cell therapy; childhood leukemia; relapse.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Disclosure of Conflict of Interest: The authors declare no conflicts of interest.
Figures
References
-
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. - PMC - PubMed
-
- Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, Ludwig WD, Ritter J, Harbott J, Mann G, Klingebiel T, Zintl F, Niemeyer C, Kremens B, Niggli F, Niethammer D, Welte K, Stanulla M, Odenwald E, Riehm H, Schrappe M. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. - PubMed
-
- Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L, Cole PD, Kelly KM, Laverdiere C, Michon B, Schorin MA, Schwartz CL, O’Holleran EW, Neuberg DS, Cohen HJ, Sallan SE. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:320–334. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical