Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate
- PMID: 25925259
- PMCID: PMC4469888
- DOI: 10.1152/ajprenal.00488.2014
Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate
Abstract
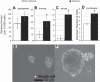
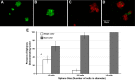
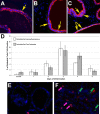
Prostatic inflammation is a nearly ubiquitous pathological feature observed in specimens from benign prostate hyperplasia and prostate cancer patients. The microenvironment of the inflamed prostate is highly reactive, and epithelial hyperplasia is a hallmark feature of inflamed prostates. How inflammation orchestrates epithelial proliferation as part of its repair and recovery action is not well understood. Here, we report that a novel epithelial progenitor cell population is induced to expand during inflammation. We used sphere culture assays, immunofluorescence, and flow cytometry to show that this population is increased in bacterially induced inflamed mouse prostates relative to naïve control prostates. We confirmed from previous reports that this population exclusively possesses the ability to regrow entire prostatic structures from single cell culture using renal grafts. In addition, putative progenitor cells harvested from inflamed animals have greater aggregation capacity than those isolated from naïve control prostates. Expansion of this critical cell population requires IL-1 signaling, as IL-1 receptor 1-null mice exhibit inflammation similar to wild-type inflamed animals but exhibit significantly reduced progenitor cell proliferation and hyperplasia. These data demonstrate that inflammation promotes hyperplasia in the mouse prostatic epithelium by inducing the expansion of a selected epithelial progenitor cell population in an IL-1 receptor-dependent manner. These findings may have significant impact on our understanding of how inflammation promotes proliferative diseases such as benign prostatic hyperplasia and prostate cancer, both of which depend on expansion of cells that exhibit a progenitor-like nature.
Keywords: hyperplasia; inflammation; interleukin-1; progenitor; prostate.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Characterization of autoimmune inflammation induced prostate stem cell expansion.Prostate. 2015 Oct;75(14):1620-31. doi: 10.1002/pros.23043. Epub 2015 Jul 14. Prostate. 2015. PMID: 26174474 Free PMC article.
-
The common parasite Toxoplasma gondii induces prostatic inflammation and microglandular hyperplasia in a mouse model.Prostate. 2017 Jul;77(10):1066-1075. doi: 10.1002/pros.23362. Epub 2017 May 12. Prostate. 2017. PMID: 28497488 Free PMC article.
-
Coordinated induction of cell survival signaling in the inflamed microenvironment of the prostate.Prostate. 2016 Jun;76(8):722-34. doi: 10.1002/pros.23161. Epub 2016 Feb 24. Prostate. 2016. PMID: 27088546 Free PMC article.
-
Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review.
-
Prostate luminal progenitor cells: from mouse to human, from health to disease.Nat Rev Urol. 2022 Apr;19(4):201-218. doi: 10.1038/s41585-021-00561-2. Epub 2022 Jan 25. Nat Rev Urol. 2022. PMID: 35079142 Review.
Cited by
-
A randomized placebo-controlled study: Phellodendron Bawei tablets combined with standard management can improve storage symptoms, sleep quality, and medication compliance in patients with benign prostatic hyperplasia compared to placebo with standard management.Transl Androl Urol. 2021 Aug;10(8):3423-3431. doi: 10.21037/tau-21-588. Transl Androl Urol. 2021. PMID: 34532267 Free PMC article.
-
Prostatic osteopontin expression is associated with symptomatic benign prostatic hyperplasia.Prostate. 2020 Jul;80(10):731-741. doi: 10.1002/pros.23986. Epub 2020 May 1. Prostate. 2020. PMID: 32356572 Free PMC article.
-
Ras/ERK and PI3K/AKT signaling differentially regulate oncogenic ERG mediated transcription in prostate cells.PLoS Genet. 2021 Jul 27;17(7):e1009708. doi: 10.1371/journal.pgen.1009708. eCollection 2021 Jul. PLoS Genet. 2021. PMID: 34314419 Free PMC article.
-
Antagonists of growth hormone-releasing hormone inhibit proliferation induced by inflammation in prostatic epithelial cells.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1359-1364. doi: 10.1073/pnas.1620884114. Epub 2017 Jan 25. Proc Natl Acad Sci U S A. 2017. PMID: 28123062 Free PMC article.
-
Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia.Urol Clin North Am. 2016 Aug;43(3):279-88. doi: 10.1016/j.ucl.2016.04.012. Urol Clin North Am. 2016. PMID: 27476121 Free PMC article. Review.
References
-
- Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol 23: 5–10, 2013. - PubMed
-
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65: 10946–10951, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

