Reduced levels of circulating progenitor cells in juvenile idiopathic arthritis are counteracted by anti TNF-α therapy
- PMID: 25925313
- PMCID: PMC4418050
- DOI: 10.1186/s12891-015-0555-9
Reduced levels of circulating progenitor cells in juvenile idiopathic arthritis are counteracted by anti TNF-α therapy
Abstract
Background: Endothelial progenitor cells (EPC) promote angiogenesis and vascular repair. Though reduced EPC levels have been shown in rheumatoid arthritis, no study has so far evaluated EPCs in children with juvenile idiopathic arthritis (JIA). We aimed to study circulating EPCs in children with JIA, their relation to disease activity, and effects of anti TNF-α treatment.
Methods: Circulating EPCs were quantified by flow cytometry based on CD34, CD133 and KDR expression in peripheral blood of 22 patients with oligoarticular JIA and 29 age-matched controls. EPCs were re-assessed in children with methotrexate-resistant oligo-extended JIA before and up to 12 month after initiation of anti-TNF-alpha therapy. Plasma concentrations of inflammatory and EPC-regulating factors were measured using a multiplex array. Confocal immunofluorescence was used to demonstrate EPCs in synovial tissues.
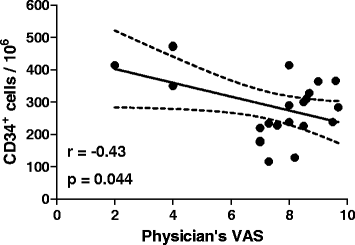
Results: Children with active JIA showed a significant reduction of relative and absolute counts of circulating progenitor cells and EPCs compared to age-matched healthy controls. CD34(+) cell levels were modestly and inversely correlated to disease activity. A strong inverse correlation was found between serum TNF-α and EPC levels. In 8 patients treated with anti TNF-α agents, the number of EPCs rose to values similar to healthy controls. CD34(+)KDR(+) EPCs were found in the synovial tissue of JIA children, but not in control.
Conclusions: Children with JIA have reduced levels of the vasculoprotective and proangiogenic EPCs. While EPCs may contribute to synovial tissue remodelling, EPC pauperization may indicate an excess cardiovascular risk if projected later in life.
Figures



Similar articles
-
Endothelial progenitor cell levels in juvenile idiopathic arthritis patients: effects of anti-inflammatory therapies.Pediatr Rheumatol Online J. 2015 Feb 19;13:6. doi: 10.1186/s12969-015-0001-4. eCollection 2015. Pediatr Rheumatol Online J. 2015. PMID: 25705139 Free PMC article.
-
Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients.Atherosclerosis. 2015 Feb;238(2):159-64. doi: 10.1016/j.atherosclerosis.2014.12.014. Epub 2014 Dec 11. Atherosclerosis. 2015. PMID: 25525743 Clinical Trial.
-
Effects of acute exercise on circulating endothelial and progenitor cells in children and adolescents with juvenile idiopathic arthritis and healthy controls: a pilot study.Pediatr Rheumatol Online J. 2015 Oct 12;13(1):41. doi: 10.1186/s12969-015-0038-4. Pediatr Rheumatol Online J. 2015. PMID: 26458943 Free PMC article.
-
Biomarkers of anti-angiogenic therapy in metastatic colorectal cancer (mCRC): original data and review of the literature.Z Gastroenterol. 2011 Oct;49(10):1398-406. doi: 10.1055/s-0031-1281752. Epub 2011 Sep 30. Z Gastroenterol. 2011. PMID: 21964893 Review.
-
The effects of TNF-alpha inhibitor therapy on the incidence of infection in JIA children: a meta-analysis.Pediatr Rheumatol Online J. 2019 Jan 18;17(1):4. doi: 10.1186/s12969-019-0305-x. Pediatr Rheumatol Online J. 2019. PMID: 30658717 Free PMC article. Review.
Cited by
-
IL-1 Inhibition in Systemic Juvenile Idiopathic Arthritis.Front Pharmacol. 2016 Dec 6;7:467. doi: 10.3389/fphar.2016.00467. eCollection 2016. Front Pharmacol. 2016. PMID: 27999545 Free PMC article. Review.
-
Assessment of angiogenesis-related parameters in juvenile idiopathic arthritis-associated uveitis.Mol Biol Rep. 2022 Jul;49(7):6093-6102. doi: 10.1007/s11033-022-07398-x. Epub 2022 Apr 1. Mol Biol Rep. 2022. PMID: 35359237
-
Pituitary adenylate cyclase-activating polypeptide attenuates tumor necrosis factor-α-induced apoptosis in endothelial colony-forming cells.Biomed Rep. 2017 Jul;7(1):11-16. doi: 10.3892/br.2017.917. Epub 2017 May 23. Biomed Rep. 2017. PMID: 28685053 Free PMC article.
-
Vitamin D Status in Rheumatoid Arthritis: Inflammation, Arterial Stiffness and Circulating Progenitor Cell Number.PLoS One. 2015 Aug 4;10(8):e0134602. doi: 10.1371/journal.pone.0134602. eCollection 2015. PLoS One. 2015. PMID: 26241902 Free PMC article.
References
-
- Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25(10):1991–4. - PubMed
-
- Scola MP, Imagawa T, Boivin GP, Giannini EH, Glass DN, Hirsch R, et al. Expression of angiogenic factors in juvenile rheumatoid arthritis: correlation with revascularization of human synovium engrafted into SCID mice. Arthritis Rheum. 2001;44(4):794–801. doi: 10.1002/1529-0131(200104)44:4<794::AID-ANR135>3.0.CO;2-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

