Activation and regulation of the granulation tissue derived cells with stemness-related properties
- PMID: 25925316
- PMCID: PMC4446126
- DOI: 10.1186/s13287-015-0070-9
Activation and regulation of the granulation tissue derived cells with stemness-related properties
Abstract
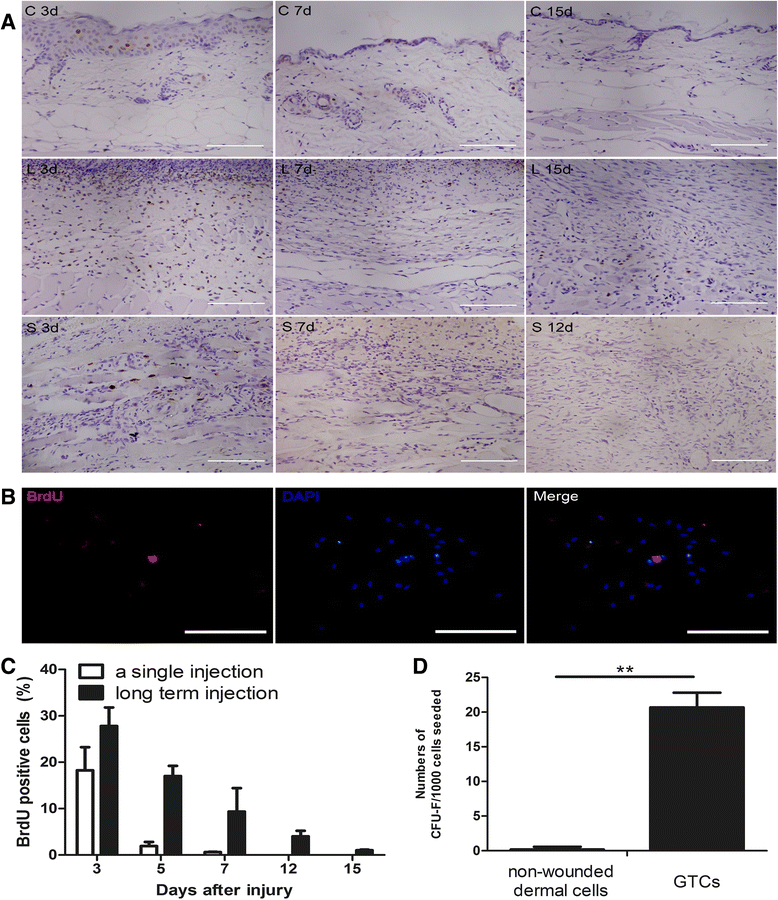
Introduction: Skin as the largest and easily accessible organ of the body represents an abundant source of adult stem cells. Among them, dermal stem cells hold great promise in tissue repair and the skin granulation tissue has been recently proposed as a promising source of dermal stem cells, but their biological characteristics have not been well investigated.
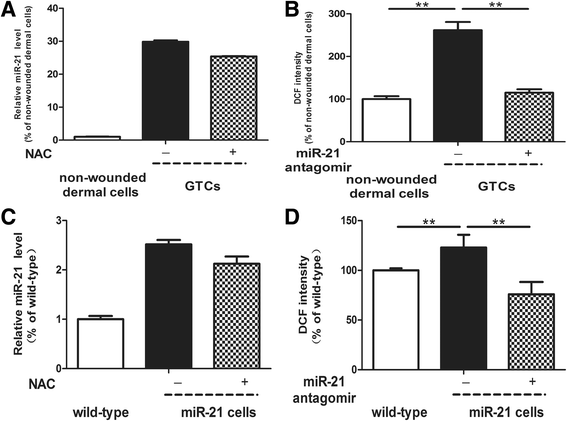
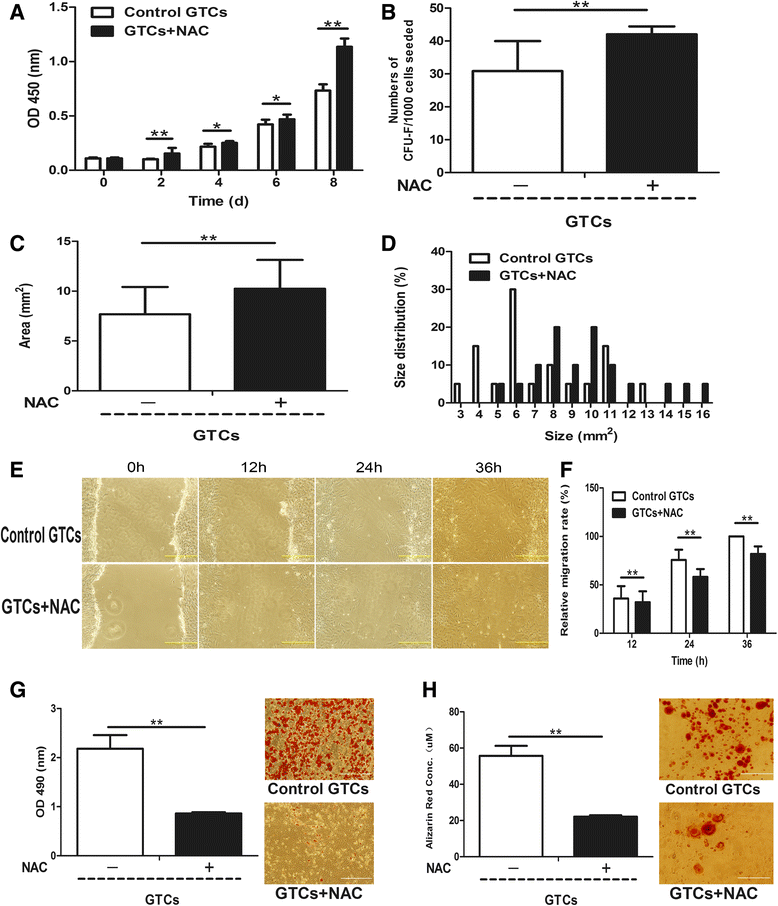
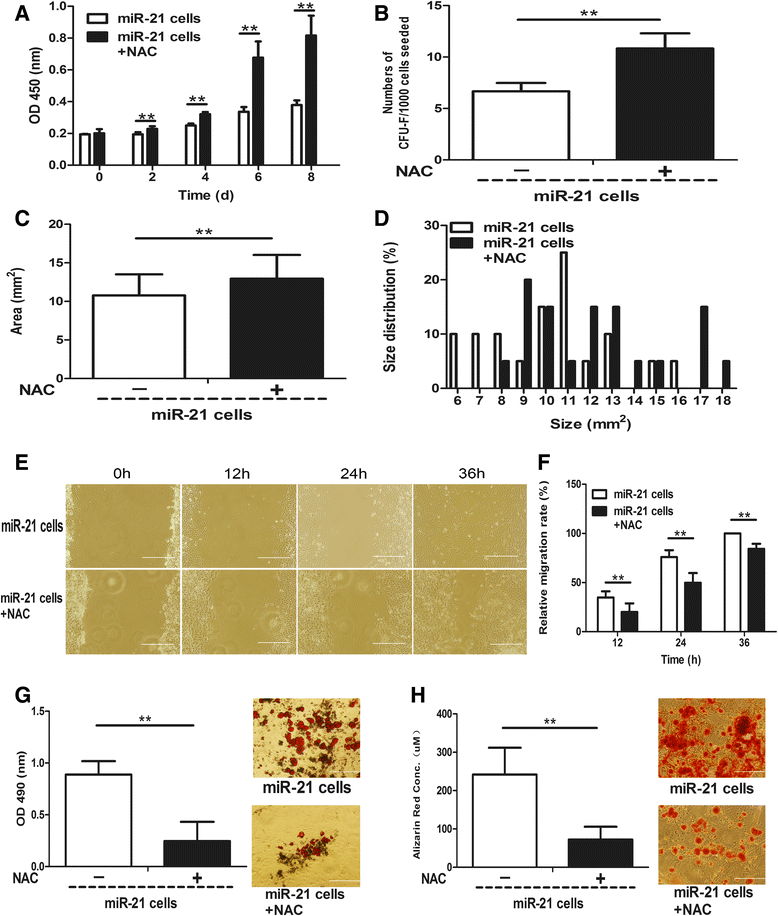
Methods: The 5-bromo-2'-deoxyuridine (BrdU) lineage tracing approach was employed to chase dermal stem cells in vivo. Granulation tissue derived cells (GTCs) were isolated and their in vitro proliferation, self-renewing, migration, and multi-differentiation capabilities were assessed. Combined radiation and skin wound model was used to investigate the therapeutic effects of GTCs. MicroRNA-21 (miR-21) antagomir was used to antagonize miR-21 expression. Reactive oxygen species (ROS) were scavenged by N-acetyl cysteine (NAC).
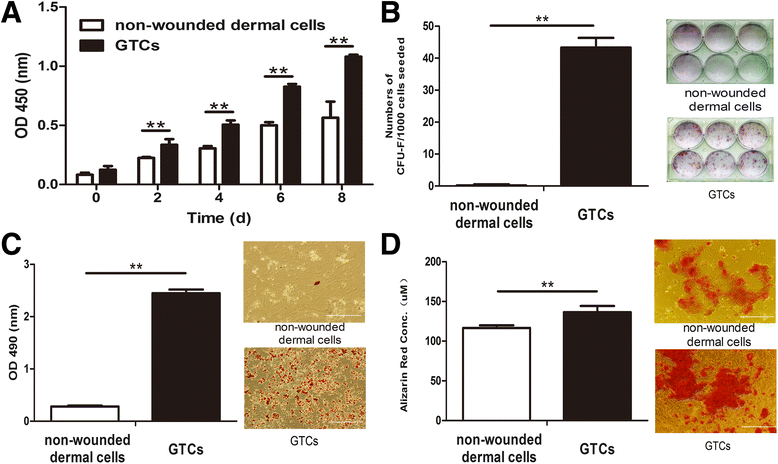
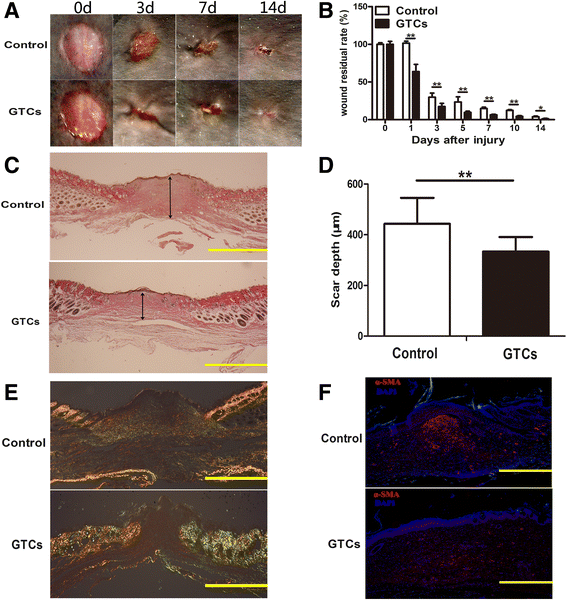
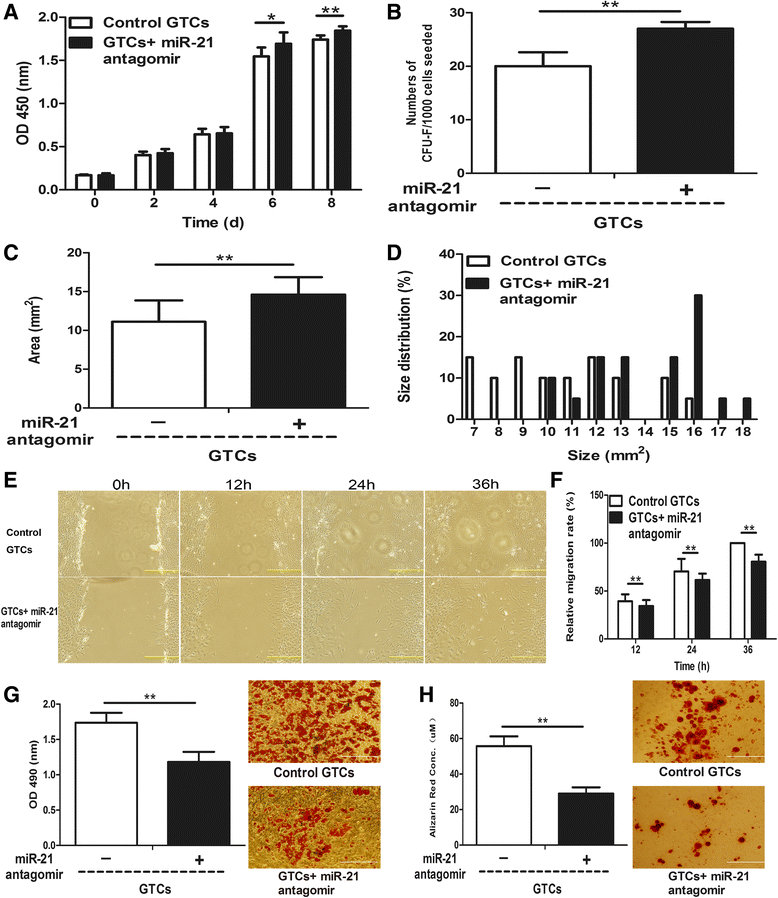
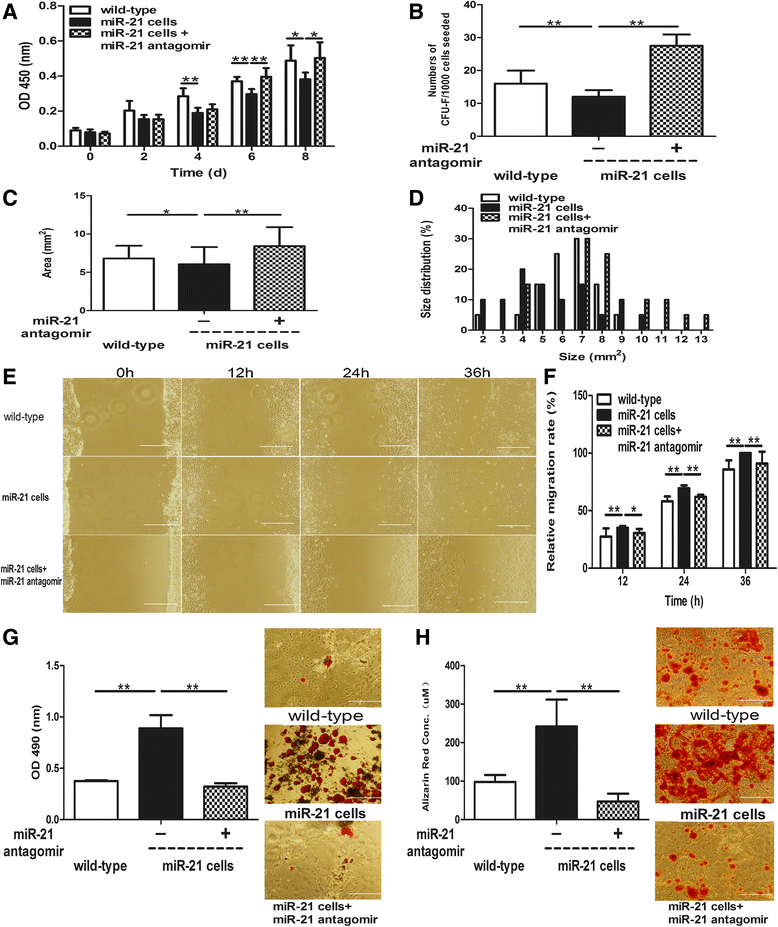
Results: The quiescent dermal stem/progenitor cells were activated to proliferate upon injury and enriched in granulation tissues. GTCs exhibited enhanced proliferation, colony formation and multi-differentiation capacities. Topical transplantation of GTCs into the combined radiation and skin wound mice accelerated wound healing and reduced tissue fibrosis. Blockade of the miR-21 expression in GTCs inhibited cell migration and differentiation, but promoted cell proliferation and self-renewing at least partially via a ROS dependent pathway.
Conclusions: The granulation tissue may represent an alternative adult stem cell source in tissue replacement therapy and miR-21 mediated ROS generation negatively regulates the stemness-related properties of granulation tissue derived cells.
Figures
References
-
- Uzarska M, Porowinska D, Bajek A, Drewa T. Epidermal stem cells – biology and potential applications in regenerative medicine. Postepy Biochem. 2013;59:219–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

